1811-28-5
Product Name:
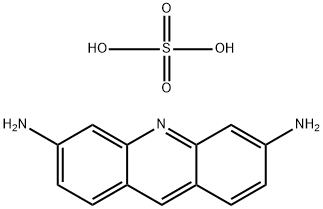
PROFLAVINE HEMISULFATE
Formula:
C13H13N3O4S
Synonyms:
3,6-Diaminoacridine hemisulfate salt
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Hygroscopic powder; [Sigma-Aldrich MSDS] |
|---|---|
| Chemical Classes | Other Uses -> Biocides/Disinfectants |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 516.6 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 0 |
| Exact Mass | 516.15797444 g/mol |
| Monoisotopic Mass | 516.15797444 g/mol |
| Topological Polar Surface Area | 213 Ų |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 313 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 3 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Proflavine Hemisulfate is the hemisulfate salt form of proflavine, an acridine-derived fluorescent contrast and disinfectant agent that can potentially be used for cellular imaging and antiseptic purposes. Upon topical application of proflavine hemisulfate, proflavine diffuses into cells and intercalates into DNA, thereby accumulating in and staining the nucleus. During fluorescence imaging, the cell nuclei can be visualized. This allows nuclear morphometry and the identification of cancer cells. In addition, proflavine exerts its antibacterial effect by binding to bacterial DNA, thereby disrupting DNA synthesis and halting bacterial cell growth.