CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Color/Form | Pale yellow needles from methylene chloride-hexane |
| Melting Point | 166-167 °C |
| Solubility | 8.58e-03 g/L |
| LogP | 2.06 |
| Stability/Shelf Life | Study has been undertaken to determine the stability of four benzodiazepines: clonazepam, midazolam, flunitrazepam & oxazepam in whole blood samples. Spiked blood was stored at four different temperatures (room temperature, 4 degrees C, -20 degrees C & -80 degrees C) & analysed at selected times during 1 yr. Determination was performed on the first, third & seventh day during the first wk, then once/wk for 3 wk, once every 2 wk for 4 wk, then once/month for 4 months & finally, once every 2 months. Extraction was performed using liquid-liquid extraction with 1-chlorobutane, while quantification was carried out using high performance liquid chromatography equipped with a photodiode-array ultraviolet detector. At room temperature, the concn of all benzodiazepines decreased over 1 yr to 100 & 70% for low & high concns, respectively. At 4 °C, the decr was between 90 & 100% for low concns & between 50 & 80% for high concns. At -20 °C, the measured decr was between 10 & 20% for high & low concns, respectively. At -80 °C, the measured loss was not significant at high concn except for midazolam. However, at low concn the determined decr was between 5 & 12%. The data collected suggests that quantitative results concerning long-term stored samples should be interpreted with caution in forensic cases. Further investigations concerning the stability of drugs in whole blood or other biological samples, additional methods of identification & determination as well as the establishment of optimal storage conditions should be undertaken in forensic cases. |
| Decomposition | When heated to decomposition it emits toxic fumes of /fluoride & nitrogen oxides/. |
| Collision Cross Section | 168.6 Ų [M+H]+ [CCS Type: TW, Method: Major Mix IMS/Tof Calibration Kit (Waters)] |
| Kovats Retention Index | 2594 2572 2572 2600 2600 2633 2571 2610 2646 2645 2633 2645 2616.4 2577 2607 2645 2611.1 |
| Other Experimental Properties | When mixed in drinks ... colorless, tasteless, odorless ... manufactured now as an oblong olive green tablet with a speckled blue core that when dissolved in light-colored /drinks/ will dye the liquid blue. Generic versions may not contain the dye |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 313.28 g/mol |
|---|---|
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 1 |
| Exact Mass | 313.08626942 g/mol |
| Monoisotopic Mass | 313.08626942 g/mol |
| Topological Polar Surface Area | 78.5 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 519 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
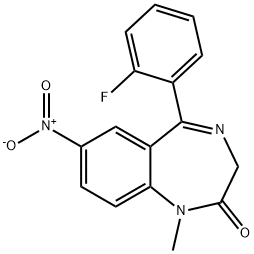
description
Flunitrazepam is a 1,4-benzodiazepinone that is nitrazepam substituted by a methyl group at position 1 and by a fluoro group at position 2'. It is a potent hypnotic, sedative, and amnestic drug used to treat chronic insomnia. It has a role as a sedative, a GABAA receptor agonist and an anxiolytic drug. It is a 1,4-benzodiazepinone, a C-nitro compound and a member of monofluorobenzenes.