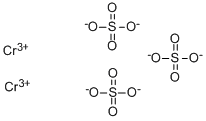
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Chromic sulfate is a dark green to violet crystalline material. Used in paints and inks, ceramics, and in textile dyeing. It is noncombustible. The primary hazard of this material is the potential for environmental damage if released. Immediate steps should be taken to limit its spread to the environment. |
|---|---|
| Color/Form | Peach-colored solid |
| Boiling Point | Loses water of hydration at 212.0 °F; Cr2(SO4)3 x 18 H2O loses 12 H2O; Cr2(SO4)3 x 15 H2O loses 10 H2O (USCG, 1999) |
| Melting Point | 212 °F (USCG, 1999) |
| Solubility | Practically insoluble in water |
| Density | 3.012 at 68 °F for anhydrous salt; Hydrated: 1.867 at 17 °C for salt with 15 H2O; 1.7 at 22 °C for salt with 18 H2O (USCG, 1999) |
| Stability/Shelf Life | Stable under recommended storage conditions./Chromium(III) sulfate hydrate/ |
| Decomposition | When heated to decomposition it emits toxic vapors of /sulfur oxides and chromium/. |
| pH | Trivalent chromium compounds are amphoteric |
| Other Experimental Properties | Hygroscopic in moist air |
| Chemical Classes | Metals -> Chromium Compounds, Inorganic |
COMPUTED DESCRIPTORS
| Molecular Weight | 392.2 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 0 |
| Exact Mass | 391.736198 g/mol |
| Monoisotopic Mass | 391.736198 g/mol |
| Topological Polar Surface Area | 266 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 62.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 5 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Chromic sulfate is a dark green to violet crystalline material. Used in paints and inks, ceramics, and in textile dyeing. It is noncombustible. The primary hazard of this material is the potential for environmental damage if released. Immediate steps should be taken to limit its spread to the environment.