SAFETY INFORMATION
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H317:Sensitisation, Skin H334:Sensitisation, respiratory H350:Carcinogenicity H372:Specific target organ toxicity, repeated exposure H400:Hazardous to the aquatic environment, acute hazard H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P221:Take any precaution to avoid mixing with combustibles/… P273:Avoid release to the environment. P308+P313:IF exposed or concerned: Get medical advice/attention. |
COMPUTED DESCRIPTORS
| Molecular Weight | 174.687 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 173.855505 g/mol |
| Monoisotopic Mass | 173.855505 g/mol |
| Topological Polar Surface Area | 80.3 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 62.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
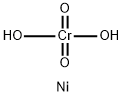
PRODUCT INTRODUCTION
description
Nickel chromate is a chemical compound of nickel and chromium. Nickel is a chemical compound with the atomic number 28. It is found abundantly in nature in laterite ore minerals, such as limonite, garnierite, and pentlandite. Nickel has a biological role and is found in certain enzymes, including urease, hydrogenase, methylcoenzyme M reductase, and carbon monoxide dehydrogenase. Hexavalent chromium refers to chemical compounds that contain the element chromium in the +6 oxidation state. Chromium(VI) is more toxic than other oxidation states of the chromium atom because of its greater ability to enter cells and higher redox potential. (L16, L40, L41)