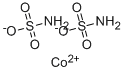CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Cobaltous sulfamate is a reddish colored solid. It is soluble in water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit the spread to the environment. It is used as a pigment and electroplating metals. |
|---|---|
| Color/Form | Reddish colored solid |
| Odor | Odorless |
| Solubility | Soluble in water |
| pH | Salts, basic, such as cobaltous sulfamate ... resulting aqueous solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. |
| Other Experimental Properties | Powder; Hygroscopic; Soluble in water /Cobalt(II) sulfamate hydrate/ |
| Chemical Classes | Metals -> Metals, Inorganic Compounds |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H341:Germ cell mutagenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. |
COMPUTED DESCRIPTORS
| Molecular Weight | 251.11 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 0 |
| Exact Mass | 250.884272 g/mol |
| Monoisotopic Mass | 250.884272 g/mol |
| Topological Polar Surface Area | 183 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 79.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 3 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Cobaltous sulfamate is a reddish colored solid. It is soluble in water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit the spread to the environment. It is used as a pigment and electroplating metals.