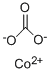CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Dry Powder; Water or Solvent Wet Solid |
|---|---|
| Color/Form | Red powder or rhombohedral crystals |
| Melting Point | 280 °C (decomposes) |
| Solubility | In water, 0.00014 g/100 g water at 20 °C |
| Density | 4.2 g/cu cm |
| Stability/Shelf Life | Stable under recommended storage conditions. /Cobalt(II) carbonate hydrate/ |
| Decomposition | When heated to decomposition it emits toxic fumes of Co |
| Refractive Index | Index of refraction: 1.855, 1.60 |
| Other Experimental Properties | Does not react with cold concentrated nitric or hydrochloric acids; when heated, dissolves with evolution of carbon dioxide; oxidized by air on weak oxidizing agents to cobalt(III) carbonate |
| Chemical Classes | Metals -> Metals, Inorganic Compounds |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin H334:Sensitisation, respiratory H341:Germ cell mutagenicity H350:Carcinogenicity H360:Reproductive toxicity H400:Hazardous to the aquatic environment, acute hazard H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P284:Wear respiratory protection. P405:Store locked up. |
COMPUTED DESCRIPTORS
| Molecular Weight | 118.942 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 118.917937 g/mol |
| Monoisotopic Mass | 118.917937 g/mol |
| Topological Polar Surface Area | 63.2 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 18.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Cobalt(II) carbonate is a chemical compound of cobalt. It occurs naturally as the mineral spherocobaltite and is used in pottery glazes. Cobalt is a metallic element with the atomic number 27. It is found naturally in rocks, soil, water, plants, and animals. In small amounts cobalt is an essential element for life, as it is part of vitamin B12. However, excess exposure is known to exhibit toxic effects. (L29, L30, L31)