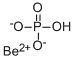CHEMICAL AND PHYSICAL PROPERTIES
| Color/Form | Crystals |
|---|---|
| Melting Point | White solid. MP: 100 °C (loses water) (decomposes). Soluble in water, acetic acid. /Beryllium phosphate trihydrate/ |
| Solubility | Insoluble in water |
| Other Experimental Properties | Loses 1 H2O at 100 °C, soluble in water and acetic acid /Trihydrate/ |
| Chemical Classes | Metals -> Beryllium Compounds, Inorganic |
COMPUTED DESCRIPTORS
| Molecular Weight | 104.992 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 104.9734286 g/mol |
| Monoisotopic Mass | 104.9734286 g/mol |
| Topological Polar Surface Area | 83.4 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 46.5 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Beryllium phosphate is a phospate of beryllium. Beryllium is a lightweight alkaline earth metal with the atomic number 4. It is a relatively rare element found naturally only combined with other elements in minerals. (L24)