13454-78-9
Product Name:
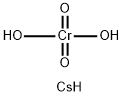
CESIUM CHROMATE
Formula:
CrCsH3O4
Synonyms:
Caesium chromate;Chromic acid cesium salt
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Yellow odorless powder; Soluble in water; [MSDSonline] |
|---|---|
| Chemical Classes | Metals -> Chromium Compounds, Inorganic |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H317:Sensitisation, Skin H350:Carcinogenicity H400:Hazardous to the aquatic environment, acute hazard H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P220:Keep/Store away from clothing/…/combustible materials. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P333+P313:IF SKIN irritation or rash occurs: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
COMPUTED DESCRIPTORS
| Molecular Weight | 381.805 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 381.731067 g/mol |
| Monoisotopic Mass | 381.731067 g/mol |
| Topological Polar Surface Area | 80.3 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 0 |
| Complexity | 62.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 3 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Cesium chromate is a chemical compound of caesium and hexavalent chromium. It is used in creating vacuum tubes. Hexavalent chromium refers to chemical compounds that contain the element chromium in the +6 oxidation state. Chromium(VI) is more toxic than other oxidation states of the chromium atom because of its greater ability to enter cells and higher redox potential. (L16, L18)
