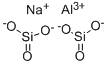CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Sodium aluminosilicate is a fine white powder. Many ordinary rocks (feldspars) are aluminosilicates. Aluminosilicates with more open three-dimensional structures than the feldspars are called zeolites. The openings in zeolites appear as polyhedral cavities connected by tunnels. Zeolites act as catalysts by absorbing small molecules in their interior cavities and holding them in proximity so that reaction among them occurs sooner. |
|---|---|
| Color/Form | FINE, WHITE, AMORPHOUS POWDER OR BEADS |
| Odor | ODORLESS |
| Taste | TASTELESS |
| Solubility | Insoluble (NTP, 1992) |
| Decomposition | When heated to decomposition ... emits toxic fumes of /disodium oxide/. |
| pH | 6.5-10.5 (20% SLURRY) |
| Chemical Classes | Metals -> Metals, Inorganic Compounds |
COMPUTED DESCRIPTORS
| Molecular Weight | 202.14 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 0 |
| Exact Mass | 201.8946485 g/mol |
| Monoisotopic Mass | 201.8946485 g/mol |
| Topological Polar Surface Area | 126 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 18.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 4 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Sodium aluminosilicate is a fine white powder. Many ordinary rocks (feldspars) are aluminosilicates. Aluminosilicates with more open three-dimensional structures than the feldspars are called zeolites. The openings in zeolites appear as polyhedral cavities connected by tunnels. Zeolites act as catalysts by absorbing small molecules in their interior cavities and holding them in proximity so that reaction among them occurs sooner.