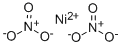CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Nickel nitrate is a green crystalline solid. It is soluble in water. It is noncombustible, but it will accelerate the burning of combustible materials. If large quantities are involved in a fire or the combustible material is finely divided, an explosion may result. Prolonged exposure to fire or heat may result in an explosion. Toxic oxides of nitrogen are produced in fires involving this material. It is used in nickel plating and to make nickel catalysts for use in chemical manufacture. |
|---|---|
| Color/Form | GREEN CRYSTALS |
| Solubility | SOL IN 238.5 G/100 CC WATER AT 0 °C /HEXAHYDRATE/ |
| Density | 2.05 at 68 °F (USCG, 1999) - Denser than water; will sink |
| Decomposition | NICKEL NITRATE SHOWED GREATER WT LOSS THAN THAT BASED ON AN OXIDE YIELD, INDICATING THAT IT OR ITS PRODUCT OF DECOMP IS VOLATILE BELOW 1100 DEG. |
| Other Experimental Properties | STRONG OXIDIZING AGENT /HEXAHYDRATE/ |
| Chemical Classes | Metals -> Nickel Compounds, Inorganic |
COMPUTED DESCRIPTORS
| Molecular Weight | 182.70 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 0 |
| Exact Mass | 181.910977 g/mol |
| Monoisotopic Mass | 181.910977 g/mol |
| Topological Polar Surface Area | 126 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 18.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 3 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Nickel nitrate is a green crystalline solid. It is soluble in water. It is noncombustible, but it will accelerate the burning of combustible materials. If large quantities are involved in a fire or the combustible material is finely divided, an explosion may result. Prolonged exposure to fire or heat may result in an explosion. Toxic oxides of nitrogen are produced in fires involving this material. It is used in nickel plating and to make nickel catalysts for use in chemical manufacture.