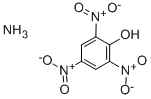
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Ammonium picrate, wetted with not less than 10% water appears as a slurry or sludge of yellow crystals in water. Will burn, although may be difficult to ignite. Produces toxic oxides of nitrogen during combustion. |
|---|---|
| Color/Form | Bright yellow scales or orthorhombic crystals; "red modification" is not a distinct polymorph, but a slightly contaminated form of the yellow salt. |
| Taste | Bitter |
| Melting Point | Decomposes |
| Solubility | Slightly soluble in alcohol |
| Density | 1.719 at 68 °F (USCG, 1999) - Denser than water; will sink |
| LogP | -1.4 |
| Decomposition | When heated to decomposition it emits highly toxic fumes of /nitroxides/. |
| Other Experimental Properties | Powerful oxidizer; when heated to decomposition it emits highly toxic fumes of NOx. |
| Chemical Classes | Other Uses -> Explosives |
COMPUTED DESCRIPTORS
| Molecular Weight | 246.13 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 0 |
| Exact Mass | 246.02364854 g/mol |
| Monoisotopic Mass | 246.02364854 g/mol |
| Topological Polar Surface Area | 162 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 292 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Ammonium picrate, wetted with not less than 10% water appears as a slurry or sludge of yellow crystals in water. Will burn, although may be difficult to ignite. Produces toxic oxides of nitrogen during combustion.