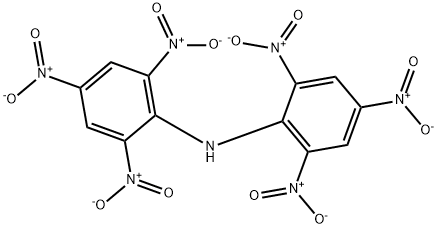
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Dipicrylamine appears as a yellow solid. Decomposes violently at high temperatures. Insoluble in water and alcohol. Used as a booster explosive. May explode under prolonged exposure to heat or fire. Primary hazard is the blast effect of an instantaneous explosion and not flying projectiles and fragments. |
|---|---|
| Color/Form | Pale yellow prisms from acetic acid |
| Melting Point | 244 °C (decomposes) |
| Solubility | Soluble in alkalies, glacial acetic acid; insoluble in acetone, alcohol, ether |
| Stability/Shelf Life | ... Extremely stable ... |
| Decomposition | When heated to decomposition it emits toxic fumes of /nitroxides/. /Nitrates/ |
| Chemical Classes | Nitrogen Compounds -> Other Aromatics (Nitrogen) |
COMPUTED DESCRIPTORS
| Molecular Weight | 439.21 g/mol |
|---|---|
| XLogP3 | 3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 13 |
| Rotatable Bond Count | 2 |
| Exact Mass | 438.99961862 g/mol |
| Monoisotopic Mass | 438.99961862 g/mol |
| Topological Polar Surface Area | 287 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 662 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Dipicrylamine appears as a yellow solid. Decomposes violently at high temperatures. Insoluble in water and alcohol. Used as a booster explosive. May explode under prolonged exposure to heat or fire. Primary hazard is the blast effect of an instantaneous explosion and not flying projectiles and fragments.