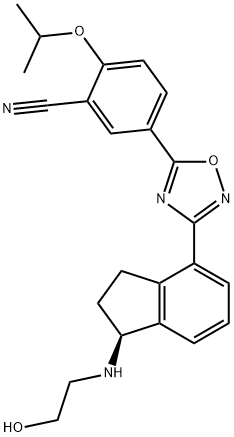
CHEMICAL AND PHYSICAL PROPERTIES
| Boiling Point | 648.3±65.0 |
|---|---|
| Melting Point | 134-137 |
| LogP | 3.73 |
| Dissociation Constants | 14.73±0.10 |
COMPUTED DESCRIPTORS
| Molecular Weight | 404.5 g/mol |
|---|---|
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 7 |
| Exact Mass | 404.18484064 g/mol |
| Monoisotopic Mass | 404.18484064 g/mol |
| Topological Polar Surface Area | 104 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 609 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Ozanimod is a once-daily sphingosine 1-phosphate receptor modulator for the treatment of relapsing Multiple Sclerosis (MS) and inflammatory bowel disease. It was developed by Celgene (now acquired by Bristol-Myers Squibb) and was approved by the FDA on March 26, 2020. The US approval was followed by the approval in Canada on October 2, 2020. In November 2021, ozanimod was also approved by the European Commission for the treatment of adults with relapsing remitting multiple sclerosis. MS is a devastating inflammatory disease that often progresses and causes severe neurological, physical, and cognitive effects. Inflammatory bowel disease also a chronic inflammatory condition and can cause persistent abdominal pain, diarrhea, bloody stools, and vomiting. In clinical trials, Ozanimod has been shown to be well-tolerated and has resulted in a higher decrease in the rate of MS relapses than with intramuscular [interferon beta-1a], a current standard in MS therapy. Studies involving patients with inflammatory bowel disease have also shown promising results.