12125-02-9
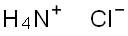
Product Name:
Ammonium chloride
Formula:
ClH4N
Synonyms:
Ammonium chloride;Ammonium chloride solution;Sal ammoniac;Salmiac;NH?Cl
Inquiry
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
PRODUCT INTRODUCTION
Description
Ammonium chloride (NH₄Cl) is a white, odorless, crystalline salt that is highly soluble in water, producing a weakly acidic solution. In medicine, NH₄Cl is an intravenous acidifier used to treat metabolic alkalosis and low chloride levels (hypochloremia). It is also used in the manufacture of dry cell batteries and ammonia compounds, as a soldering flux, a pickling agent for zinc and tin plating, and as a fertilizer.
