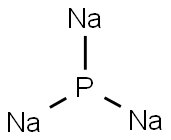
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Sodium phosphide appears as a red solid. |
|---|---|
| Color/Form | Red solid |
| Melting Point | >650 °C |
| Solubility | Reacts with water |
| Density | 1.74 g/cu cm |
| Decomposition | When heated to deomposition it emits toxic fumes of /phosphorus and sodium oxides/. |
| Other Experimental Properties | Decomposes on heating and in water, forming phosphine |
| Chemical Classes | Metals -> Phosphides |
COMPUTED DESCRIPTORS
| Molecular Weight | 99.9430698 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 99.94306984 g/mol |
| Monoisotopic Mass | 99.94306984 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 4 |
| Formal Charge | 0 |
| Complexity | 0 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 4 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Sodium phosphide appears as a red solid.