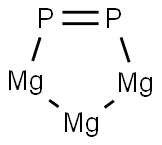
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Magnesium phosphide is a white crystalline solid. It reacts violently with water and may ignite upon contact with air. It is toxic by ingestion. It is used to make other chemicals. |
|---|---|
| Color/Form | Yellow cubic crystals |
| Melting Point | >750 °C |
| Solubility | Reacts with water |
| Density | 2.06 g/cu cm |
| Decomposition | When heated to decomposition it emits toxic fumes of /phosphorus oxides/ and phosphine. |
| Relative Evaporation Rate | Evaporation at 20 °C is negligible |
| Other Experimental Properties | Produces spontaneously flammable phosphine and diphosphine on contact with water. |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H227:Flammable liquids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. P370+P378:In case of fire: Use … for extinction. P405:Store locked up. P403+P233:Store in a well-ventilated place. Keep container tightly closed. P403+P235:Store in a well-ventilated place. Keep cool. |
COMPUTED DESCRIPTORS
| Molecular Weight | 134.86 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 133.9026491 g/mol |
| Monoisotopic Mass | 133.9026491 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 0 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 5 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Magnesium phosphide is a white crystalline solid. It reacts violently with water and may ignite upon contact with air. It is toxic by ingestion. It is used to make other chemicals.