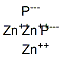CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Zinc phosphide is a dark gray granular solid. It is slowly decomposed by water giving off phosphine, a flammable poison gas. It is toxic by ingestion. It is used in medicine and as a rat poison. |
|---|---|
| Color/Form | Dark gray tetragonal crystals; lustrous or dull powder |
| Odor | Faint phosphorus odor |
| Boiling Point | 2012 °F at 760 mmHg (EPA, 1998) |
| Melting Point | 788 °F (EPA, 1998) |
| Solubility | Practically insoluble in water, decomposes slowly |
| Density | 4.55 (EPA, 1998) - Denser than water; will sink |
| Vapor Pressure | Negligible in the dry state |
| Stability/Shelf Life | Stable under recommended storage conditions. |
| Decomposition | Hazardous decomposition products formed under fire conditions - Oxides of phosphorus, zinc/zinc oxides. |
| Corrosivity | Slightly corrosive to metals |
| Heat of Combustion | -4,100 Btu/lb= -2,270 cal/g= -95X10+5 J/kg |
| Other Experimental Properties | Reacts violently with water and acids to liberate phosphine, a flammable and toxic gas. Stable when dry. |
| Chemical Classes | Metals -> Phosphides |
COMPUTED DESCRIPTORS
| Molecular Weight | 258.1 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 255.73184 g/mol |
| Monoisotopic Mass | 253.73495 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 0 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 5 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Zinc phosphide is a dark gray granular solid. It is slowly decomposed by water giving off phosphine, a flammable poison gas. It is toxic by ingestion. It is used in medicine and as a rat poison.