1113-60-6
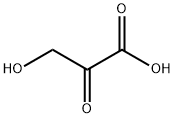
Product Name:
β-Hydroxypyruvic acid
Formula:
C3H4O4
Synonyms:
3-Hydroxy-2-oxopropanoic acid
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Solubility | soluble in water at 50 g/l at 20�C and white petrolatum at <100 g/kg at 20�C |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 104.06 g/mol |
|---|---|
| XLogP3 | -1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 2 |
| Exact Mass | 104.01095860 g/mol |
| Monoisotopic Mass | 104.01095860 g/mol |
| Topological Polar Surface Area | 74.6 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 0 |
| Complexity | 95.1 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
3-hydroxypyruvic acid is a 2-oxo monocarboxylic acid that is pyruvic acid in which one of the methyl hydrogens is substituted by a hydroxy group. It is an intermediate involved in the glycine and serine metabolism. It has a role as a human metabolite and an Escherichia coli metabolite. It is a 2-oxo monocarboxylic acid, a 3-hydroxy monocarboxylic acid and a primary alpha-hydroxy ketone. It is functionally related to a pyruvic acid. It is a conjugate acid of a 3-hydroxypyruvate.
