CHEMICAL AND PHYSICAL PROPERTIES
| Boiling Point | Decomposes at 214 ºC |
|---|---|
| Melting Point | > 214 ºC |
| Solubility | 0.02 mg/ml (in the form of di-HCl monohydrate salt) |
| LogP | 2.28 |
| Caco2 Permeability | 5.98e-06 |
| Dissociation Constants | 6 |
COMPUTED DESCRIPTORS
| Molecular Weight | 374.4 g/mol |
|---|---|
| XLogP3 | 2.4 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 374.18550935 g/mol |
| Monoisotopic Mass | 374.18550935 g/mol |
| Topological Polar Surface Area | 96.8 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 595 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
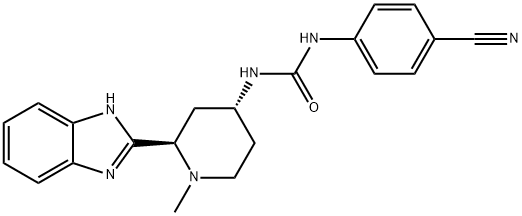
Glasdegib is a member of the class of benzimidazoles that is 1H-benzimidazole which is substituted by a (2R,4S)-4-{[(4-cyanophenyl)carbamoyl]amino}-1-methylpiperidin-2-yl group at position 2. It is a hedgehog signalling pathway inhibitor that acts by binding to Smoothened (SMO) receptors and blocking signal transduction (IC50 = 5 nM). It is used in combination with low-dose cytarabine, for the treatment of newly-diagnosed acute myeloid leukemia (AML) in adult patients (aged >= 75 years), or who have medical conditions that prevent the use of standard chemotherapy. It has a role as a SMO receptor antagonist, a Hedgehog signaling pathway inhibitor and an antineoplastic agent. It is a member of benzimidazoles, a member of piperidines, a member of phenylureas and a nitrile.