108-31-6
Product Name:
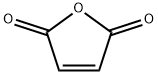
Maleic anhydride
Formula:
C4H2O3
Synonyms:
2,5-Furandione;2,5-Furanedione;Maleic anhydride
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Maleic anhydride appears as colorless crystalline needles, flakes, pellets, rods, briquettes, lumps or a fused mass. Melts at 113 °F. Shipped both as a solid and in the molten state. Vapors, fumes and dusts strong irritate the eyes, skin and mucous membranes. Flash point 218 °F. Autoignition temperature 890 °F. Used to make paints and plastics and other chemicals. |
|---|---|
| Color/Form | Orthorhombic needles from chloroform; commercial grades furnished in fused form, as briquettes |
| Odor | Pungent odor |
| Boiling Point | 387 to 390 °F at 760 mmHg (NTP, 1992) |
| Melting Point | 127 °F (NTP, 1992) |
| Flash Point | 218 °F (NTP, 1992) |
| Solubility | Soluble; decomposes in hot solvent (NTP, 1992) |
| Density | 1.43 at 59 °F (USCG, 1999) - Denser than water; will sink |
| Vapor Density | 3.38 (Air = 1) |
| Vapor Pressure | 0.2 mmHg (NIOSH, 2023) |
| LogP | log Kow = 1.62 |
| Stability/Shelf Life | Stable under normal laboratory storage conditions. |
| Autoignition Temperature | 878 °F (USCG, 1999) |
| Decomposition | Maleic anhydride decomposes exothermically, evolving carbon dioxide in the presence of dimethylamine, triethylamine, pyridine, or quinoline at temperatures above 150 °C. |
| Viscosity | Viscosity in mPa.s (cP): 0.61 at 60 °C; 1.07 at 90 °C; 0.6 at 150 °C |
| Corrosivity | Corrosive material. |
| Heat of Combustion | -1389.5 kJ/mol |
| Heat of Vaporization | 54.8 kJ/mol |
| pH | pH of water solutions: 2.42 at 1X10-2 M; 2.62 at 5X10-3 M; 3.10 at 1X10-4 M |
| Ionization Potential | 9.90 eV |
| Odor Threshold | Odor Threshold Low: 0.25 [mmHg] Odor Threshold High: 0.32 [mmHg] Odor threshold from AIHA |
| Kovats Retention Index | 791 807 790 |
| Other Experimental Properties | Specific heat: 0.285 (solid); 0.396 (liquid). |
| Chemical Classes | Plastics & Rubber -> Acid Anhydrides, Cyclic |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H314:Skin corrosion/irritation H317:Sensitisation, Skin H334:Sensitisation, respiratory H372:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 98.06 g/mol |
|---|---|
| XLogP3 | -0.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 98.000393922 g/mol |
| Monoisotopic Mass | 98.000393922 g/mol |
| Topological Polar Surface Area | 43.4 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 0 |
| Complexity | 129 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Maleic anhydride is a colorless crystalline solid that may appear as needles, flakes, pellets, or a fused mass, with a melting point of 113°F. It is shipped in both solid and molten forms. Its vapors, fumes, and dust are highly irritating to the eyes, skin, and mucous membranes. With a flash point of 218°F and an autoignition temperature of 890°F, it is primarily used in the production of paints, plastics, and other chemicals.
