10418-03-8
Product Name:
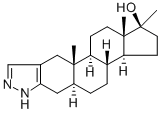
Stanozolol
Formula:
C21H32N2O
Synonyms:
17β-Hydroxy-17α-methyl-5α-androstano[3,2-c]pyrazole;5α-Androstane-17α-methyl-17β-ol-[3,2-c]pyrazole;Androstanazole;Stanazol
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Color/Form | Crystals from alcohol |
| Odor | ODORLESS |
| Melting Point | 229.8-242.0 °C |
| Solubility | INSOL IN WATER, SOL IN DIMETHYLFORMAMIDE; SPARINGLY SOL IN ALC AND CHLOROFORM; SLIGHTLY SOL IN ETHYL ACETATE AND ACETONE; VERY SLIGHTLY SOL IN BENZENE |
| Optical Rotation | Specific optical rotation: +35.7 deg/D (chloroform); +48.6 deg/D (methanol); |
| Collision Cross Section | 188.1 Ų [M+H]+ [CCS Type: TW, Method: Major Mix IMS/Tof Calibration Kit (Waters)] |
| Other Experimental Properties | There are 2 forms of the solid. The needles melt at about 155 °C and the prisms melt at 235 °C. |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H351:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
COMPUTED DESCRIPTORS
| Molecular Weight | 328.5 g/mol |
|---|---|
| XLogP3 | 4.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 328.251463648 g/mol |
| Monoisotopic Mass | 328.251463648 g/mol |
| Topological Polar Surface Area | 48.9 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 538 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 7 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Stanozolol is an organic heteropentacyclic compound resulting from the formal condensation of the 3-keto-aldehyde moiety of oxymetholone with hydrazine. Like oxymetholone, it is a synthetic anabolic steroid. It has both anabolic and androgenic properties, and has been used to treat hereditary angioedema and various vascular disorders. It has also been widely abused by professional athletes. It has a role as an androgen and an anabolic agent. It is a 17beta-hydroxy steroid, a tertiary alcohol, an anabolic androgenic steroid and an organic heteropentacyclic compound. It is functionally related to an oxymetholone.
