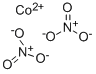
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Cobalt nitrate is an odorless red solid. Sinks and mixes with water. (USCG, 1999) |
|---|---|
| Color/Form | Pale red powder |
| Odor | Odorless |
| Melting Point | 131 °F (USCG, 1999) |
| Solubility | Solubility: 97.929 lb/100 lb water at 70 °F /Cobaltous nitrate hexahydrate/ |
| Density | 1.54 at 68 °F (USCG, 1999) - Denser than water; will sink |
| Decomposition | Decomposes at 75 °C. |
| pH | pH 4.0 at 100 g/L at 20 °C |
| Relative Evaporation Rate | Evaporation at 20 °C is negligible |
| Other Experimental Properties | Standard enthalpy of formation: -420.5 kJ/mol |
| Chemical Classes | Metals -> Metals, Inorganic Compounds |
COMPUTED DESCRIPTORS
| Molecular Weight | 182.94 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 0 |
| Exact Mass | 182.908829 g/mol |
| Monoisotopic Mass | 182.908829 g/mol |
| Topological Polar Surface Area | 126 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 18.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 3 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Cobalt nitrate is an odorless red solid. Sinks and mixes with water. (USCG, 1999)
RELATED SUPPLIERS
Sajan Overseas Private Limited
Ahmedabad
product:Powder Cobalt Nitrate, For Industrial,Laboratory, Grade: Technical Grade