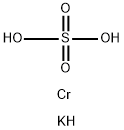
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Violet-red crystals; soluble in water; [Hawley] Red-violet odorless granules; [Mallinckrodt Baker MSDS] |
|---|---|
| Chemical Classes | Metals -> Chromium Compounds, Inorganic |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 283.22 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 0 |
| Exact Mass | 282.807670 g/mol |
| Monoisotopic Mass | 282.807670 g/mol |
| Topological Polar Surface Area | 177 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 62.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 4 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Chromium(III) potassium sulfate is a sulfate of chromium and potassium. It can be used for leather tanning. Chromium is a chemical element which has the symbol Cr and atomic number 24. It is found naturally occuring in rocks, animals, plants, and soil, and is usually mined as chromite ore. Chromium is most toxic in its +6 oxidation state (chromium(VI)) due to its greater ability to enter cells and higher redox potential. Trivalent chromium (chromium(III)) however, is biologically necessary for sugar and lipid metabolism in humans. (L17, L514)