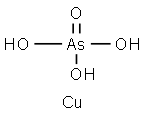
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Light blue, blue, or bluish green solid; [Hawley] Blue-green to blue insoluble powder; [Ullmann] |
|---|---|
| Chemical Classes | Metals -> Arsenic Compounds, Inorganic |
COMPUTED DESCRIPTORS
| Molecular Weight | 468.48 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 0 |
| Exact Mass | 468.58949 g/mol |
| Monoisotopic Mass | 466.59130 g/mol |
| Topological Polar Surface Area | 173 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 36.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 5 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Copper arsenate is a chemical compound of copper and arsenic. It is derived from arsenic acid and used mainly as a pesticide. Copper is a chemical element with the symbol Cu and atomic number 29. Copper is an essential elements in plants and animals as it is required for the normal functioning of more than 30 enzymes. It occurs naturally throughout the environment in rocks, soil, water, and air. Arsenic is a chemical element that has the symbol As and atomic number 33. It is a poisonous metalloid that has many allotropic forms: yellow (molecular non-metallic) and several black and grey forms (metalloids) are a few that are seen. Three metalloidal forms of arsenic with different crystal structures are found free in nature (the minerals arsenopyrite and the much rarer arsenolamprite and pararsenolamprite), but it is more commonly found as a compound with other elements. (T3, L277, L278, L380)