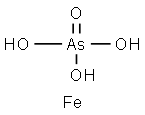
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Ferric arsenate is a green or brown powder. It is insoluble in water. It is toxic by ingestion and inhalation and is a strong irritant. It is used in the manufacture of insecticides. |
|---|---|
| Color/Form | The solid is a green or brown powder. |
| Melting Point | Melting point: decomposes /Dihydrate/ |
| Solubility | The solubility of ferric arsenate (FeAsO4) was studied at 25-73 °C and pH 3.5-11 with regard to removal of arsenic from waste waters; solubility is negligible in neutral soln. However, it was 4.4X10-6 and 3.8X10-2 g-ion/L at 73 °C and pH 3 and 11, respectively. |
| Vapor Pressure | 0.00000075 [mmHg] |
| Decomposition | Dangerous when heated to decomp ... It emits toxic fumes of arsenic /srp: including arsine/. /Arsenic cmpd/ |
| Refractive Index | Index of refraction: 1,765, 1.774, 1.797; green rhombic crystals; insoluble in water, nitric acid; soluble in hydrochloric acid /Dihydrate/ |
| Other Experimental Properties | Green or brown powder. Density 3.18, decomp on heating. Insoluble in water; sol in dilute mineral acids /Dihydrate/ |
| Chemical Classes | Metals -> Arsenic Compounds, Inorganic |
COMPUTED DESCRIPTORS
| Molecular Weight | 194.76 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 194.836189 g/mol |
| Monoisotopic Mass | 194.836189 g/mol |
| Topological Polar Surface Area | 86.2 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 36.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Ferric arsenate is a green or brown powder. It is insoluble in water. It is toxic by ingestion and inhalation and is a strong irritant. It is used in the manufacture of insecticides.