101-81-5
Product Name:
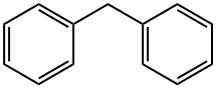
Diphenylmethane
Formula:
C13H12
Synonyms:
Benzylbenzene;Diphenylmethane;Methylenedibenzene
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Liquid |
|---|---|
| Color/Form | Orthorhombic needles |
| Odor | Odor of oranges |
| Boiling Point | 264.5 °C |
| Melting Point | 25.4 °C |
| Flash Point | 130 °C |
| Solubility | In water, 14.1 mg/L at 25 °C |
| Density | 1.3421 at 10 °C/4 °C (solid); 1.0008 at 26 °C/4 °C (liquid) |
| Vapor Density | 5.8 (Air= 1) |
| Vapor Pressure | 0.00821 [mmHg] |
| LogP | log Kow = 4.14 |
| Autoignition Temperature | 905 °F (485 °C) |
| Decomposition | When heated to decomposition it emits acrid smoke and irritating fumes. |
| Heat of Combustion | 41.2X10+3 kJ/kg at 20 °C |
| Refractive Index | Index of refraction: 1.57683 at 20 °C/D |
| Kovats Retention Index | 1380.1 1389.6 1403.9 1405.2 1448.7 1414.6 1423.5 1429.8 1412.4 1429.4 1415 1449 1424 1380.1 1400 1401 1406 1412 1416 1422.4 1403 1393 1461 |
| Chemical Classes | Other Classes -> Aromatic Hydrocarbons |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Environment GHS09 |
| GHS Hazard Statements |
H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P391:Collect spillage. Hazardous to the aquatic environment P501:Dispose of contents/container to..… |
COMPUTED DESCRIPTORS
| Molecular Weight | 168.23 g/mol |
|---|---|
| XLogP3 | 4.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 2 |
| Exact Mass | 168.093900383 g/mol |
| Monoisotopic Mass | 168.093900383 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 111 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Diphenylmethane is a diarylmethane that is methane substituted by two phenyl groups.
RELATED SUPPLIERS
All suppliers(4)
