100-88-9
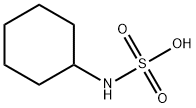
Product Name:
Cyclamic acid
Formula:
C6H13NO3S
Synonyms:
N-Cyclohexylsulfamic acid;Cyclamic acid;Cyclohexanesulfamic acid
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | White odorless solid; [Hawley] Fine white crystals; [MSDSonline] |
|---|---|
| Color/Form | Crystals |
| Odor | Odorless |
| Taste | Sweet-sour |
| Melting Point | 169.5 °C |
| Solubility | 1 G DISSOLVES IN ABOUT 7.5 ML OF WATER, 4 ML OF ALC, 4 ML OF PROPYLENE GLYCOL, OR 6 ML OF ACETONE; SLIGHTLY SOL IN CHLOROFORM AND INSOL IN HEXANE |
| Vapor Pressure | 0.0000027 [mmHg] |
| Decomposition | When heated to decomposition it emits toxic fumes of sulfoxides and nitroxides. |
| pH | pH of 10% aqueous solution: 0.8-1.6 |
| Other Experimental Properties | Sugar equivalence value relative to sucrose = 30 |
| Chemical Classes | Other Uses -> Food Additives |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
COMPUTED DESCRIPTORS
| Molecular Weight | 179.24 g/mol |
|---|---|
| XLogP3 | 0.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 2 |
| Exact Mass | 179.06161445 g/mol |
| Monoisotopic Mass | 179.06161445 g/mol |
| Topological Polar Surface Area | 74.8 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 200 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Cyclohexylsulfamic acid is a member of the class of sulfamic acids that is sulfamic acid carrying an N-cyclohexyl substituent. It has a role as a human xenobiotic metabolite and an environmental contaminant. It is functionally related to a sulfamic acid. It is a conjugate acid of a cyclohexylsulfamate.
