factory supply11113-50-1 boric acid flakes , powder, chunks
| Price | USD50.00 |
| Packge | 1KG |
- Min. Order:1KG
- Supply Ability:50ton
- Time:2021-08-07
Product Details
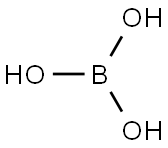
- Product Name Boric acid
- CAS No.11113-50-1
- EINECS No.200-002-5
- MFBH3O3
- MW61.83
- AppearanceLiquidClear, dark blue
- storage temp. Room Temperature
- Melting point >148oC (dec.)
| Product Name: | Boric acid |
| Synonyms: | Boric acid Manufacturer;boric acid cas 11113-50-1 factory supply kf-wang(at)chem.com;Boric acid Joyce;Boric acid test solution(ChP);Boric acid;Borsrenatliche;borid acid;BoricAcid(AS),Borofax |
| CAS: | 11113-50-1 |
| MF: | BH3O3 |
| MW: | 61.83 |
| EINECS: | 234-343-4 |
| Product Categories: | UVCBs-inorganic |
| Mol File: | 11113-50-1.mol |
| |
B in the form of borates has long been recognized as an essential plant micronutrient for the growth and viability of plants. Recently, there has been a growing body of evidence that B may be an essential element for frogs, fish, rats, and humans, as well as for plants[1-5]. The major sources of B exposure are diet and drinking water. Fruits, vegetables, and nuts are especially rich in B. Rainey et al.[6] recently studied daily dietary B intake, evaluating the food consumption records of over 25,000 Americans over several days. The median, mean, and 95 percentile B intake for all participants were 0.76, 0.93, and 2.4 mg B/d, respectively.
Boric acid (H3BO3) is a boron compound that is soluble and circulates in plasma[7]. It is a colorless, water-soluble, salt-like white powder, which have been used as pesticide since 1948. Normally, it is used to kill mites, insects, fungi and algae. For instances fleas, cockroaches, termites and wood decay fungi[8, 9]. Borate chemicals and boric acid have been used extensively for industrial purposes and its salts have been used for medication as an antiseptic to kill bacteria and fungi. Normally, it is used in the form of powder and liquid; depending to the target and conditions of pest, boric acid might applied as a spray or aerosol, as well as in the form of tablets, granule, pellets, paste or crystalline[10].
Besides that, when boric acid used as an herbicide, it desiccates or disrupts the photosynthesis system in plants. Hence, boric acid is normally used to suppress algae in swimming pools and sewage systems[8]. On the other hand, as a fungicide, the fungicidal properties of boric acid prevent the production of conidia or asexual spores of the fungi; hence, it suppresses the growth of fungi. Therefore, boric acid is used as wood preservative in wood industry such as lumber and timber products that controls decay producing fungi[9,14].
Boric acid is reported to be used as food preservatives in some foods and food products. Boric acid is used for preserving meats, meat products, caviar and dairy products[15]. This is because boric acid is able to inhibit the growth of microorganism, therefore, the preserved food can stay fresh and longer[16]. Moreover, according to Yiu et al. (2008)[17], boric acid was added to some food products to control starch gelatinization, as well as enhance the color, texture and flavor of the food.
Boric acid is used in producing the glass faceplates of LCD flat panel displays. In electroplating, boric acid is used as part of some proprietary formulas. It is also used in the manufacturing of “remming mass”, a fine silica-containing powder used for producing induction furnace linings. Borates including boric acid have been used since the time of the Greeks for cleaning, preserving food, and other activities. It is used in pyrotechnics to prevent the amide-forming reaction between aluminum and nitrates. A small amount of boric acid is added to the composition to neutralize alkaline amides that can react with the aluminum. Boric acid dissolved in methane is popularly used among fire jugglers and fire spinners to create a deep green flame. Boric acid is added to salt in the curing of cattle hides, calfskins and sheepskins. Used in that way it helps to control bacteria development and also aids in the control of insects.
Company Profile Introduction
Shijiazhuang Tongyang Import and Export Co., LTD , located in Shijiazhuang City,which is the capital of Hebei Province and hub sector among Beijing, Tianjin, and Hebei.We have the advantage of convenient transportation.Our company is a modern high-tech chemical enterprise with Research & Development, production and sales.In recent five years, the company has implemented the strategy of "going out" vigorously.We has established our branches in Hubei China, Vietnam and Mexico, making our market and sales network more and more perfect.Our company will continue to adhere to the strategic positioning of fine chemical industry in the future and competition ideas of irreplaceable product differentiation,and strive to become to the leading of Chinese chemical industry.Shijiazhuang Tongyang Import and Export Co., LTD , located in Shijiazhuang City,which is the capital of Hebei Province and hub sector among Beijing, Tianjin, and Hebei.We have the advantage of convenient transportation.Our company is a modern high-tech chemical enterprise with Research & Development, production and sales.In recent five years, the company has implemented the strategy of "going out" vigorously.We has established our branches in Hubei China, Vietnam and Mexico, making our market and sales network more and more perfect.Our company will continue to adhere to the strategic positioning of fine chemical industry in the future and competition ideas of irreplaceable product differentiation,and strive to become to the leading of Chinese chemical industry.
Recommended supplier
-
VIP1年
- S D Fine Chem Limited
- 10043-35-3 / 11113-50-1 99%
- Inquiry
- 2024-03-16
-
VIP1年
- Paras Chemical Industries
- Boric acid 99%
- Inquiry
- 2024-02-29
-
VIP1年
- Indo Borax and Chemicals Ltd
- 10043-35-3 / 11113-50-1 99%
- Inquiry
- 2024-02-24
-
VIP1年
- Gujarat Boron Derivatives Pvt Ltd
- 10043-35-3 / 11113-50-1 Boric acid 98%
- Inquiry
- 2024-02-24
-
VIP1年
- Innovacorp India Pvt Ltd
- Boric acid 10043-35-3 / 11113-50-1 99%
- Inquiry
- 2024-02-08
-
VIP1年
- Hemadri Chemicals
- 10043-35-3 / 11113-50-1 98%
- Inquiry
- 2024-02-08
-
VIP1年
- Expresolv Limited
- 10043-35-3 / 11113-50-1 Boric acid 98%
- Inquiry
- 2024-02-07
-
VIP1年
- Shriji Chemicals
- Boric acid 10043-35-3 / 11113-50-1 98%
- Inquiry
- 2024-02-06
-
VIP1年
- Nandu Chemical Industries
- Boric acid 99%
- Inquiry
- 2024-02-01
-
VIP1年
- Indenta Chemicals (India) Pvt Ltd
- 10043-35-3 / 11113-50-1 Boric acid 98%
- Inquiry
- 2024-01-19
- Since:2015-05-06
- Address: room 1916,haiyuetiandi,66 yuehua west road,xiaoxi district