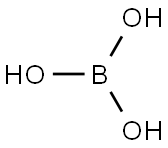
11113-50-1 boric acid flakes , powder, chunks
| Price | USD50.00 |
| Packge | 1KG |
- Min. Order:1KG
- Supply Ability:50ton
- Time:2021-08-07
Product Details
- Product Name Boric acid
- CAS No.11113-50-1
- EINECS No.200-002-5
- MFBH3O3
- MW61.83
- AppearanceLiquidClear, dark blue
- storage temp. Room Temperature
- Melting point >148oC (dec.)
| Product Name: | Boric acid |
| Synonyms: | Boric acid Manufacturer;boric acid cas 11113-50-1 factory supply kf-wang(at)chem.com;Boric acid Joyce;Boric acid test solution(ChP);Boric acid;Borsrenatliche;borid acid;BoricAcid(AS),Borofax |
| CAS: | 11113-50-1 |
| MF: | BH3O3 |
| MW: | 61.83 |
| EINECS: | 234-343-4 |
| Product Categories: | UVCBs-inorganic |
| Mol File: | 11113-50-1.mol |
| |
B in the form of borates has long been recognized as an essential plant micronutrient for the growth and viability of plants. Recently, there has been a growing body of evidence that B may be an essential element for frogs, fish, rats, and humans, as well as for plants[1-5]. The major sources of B exposure are diet and drinking water. Fruits, vegetables, and nuts are especially rich in B. Rainey et al.[6] recently studied daily dietary B intake, evaluating the food consumption records of over 25,000 Americans over several days. The median, mean, and 95 percentile B intake for all participants were 0.76, 0.93, and 2.4 mg B/d, respectively.
Boric acid (H3BO3) is a boron compound that is soluble and circulates in plasma[7]. It is a colorless, water-soluble, salt-like white powder, which have been used as pesticide since 1948. Normally, it is used to kill mites, insects, fungi and algae. For instances fleas, cockroaches, termites and wood decay fungi[8, 9]. Borate chemicals and boric acid have been used extensively for industrial purposes and its salts have been used for medication as an antiseptic to kill bacteria and fungi. Normally, it is used in the form of powder and liquid; depending to the target and conditions of pest, boric acid might applied as a spray or aerosol, as well as in the form of tablets, granule, pellets, paste or crystalline[10].
Besides that, when boric acid used as an herbicide, it desiccates or disrupts the photosynthesis system in plants. Hence, boric acid is normally used to suppress algae in swimming pools and sewage systems[8]. On the other hand, as a fungicide, the fungicidal properties of boric acid prevent the production of conidia or asexual spores of the fungi; hence, it suppresses the growth of fungi. Therefore, boric acid is used as wood preservative in wood industry such as lumber and timber products that controls decay producing fungi[9,14].
Boric acid is reported to be used as food preservatives in some foods and food products. Boric acid is used for preserving meats, meat products, caviar and dairy products[15]. This is because boric acid is able to inhibit the growth of microorganism, therefore, the preserved food can stay fresh and longer[16]. Moreover, according to Yiu et al. (2008)[17], boric acid was added to some food products to control starch gelatinization, as well as enhance the color, texture and flavor of the food.
BA is distributed similarly in humans and animals. It is rapidly distributed throughout body water. After administration of BA, B levels in soft tissues are equivalent to those found in plasma, whereas bone B levels appear to be higher than those found in plasma or soft tissues. In humans, a greater concentration of B in bone was reported relative to other tissues. Bone B concentrations were determined on 116 ashed samples from 33 human cadavers[25, 26]. More recently, Ward[27] examined B concentrations using a more sophisticated neutron activation analytical technique in a variety of human tissues, including bone, from 14 normal individuals and 18 individuals with rheumatoid arthritis. High B levels were found in bone, hair, and teeth.
BA is not metabolized in humans or animals. The metabolism of BA by biological systems is not possible owing to the high energy requirements (523 kJ/mol) needed to break the B----O bond[28]. In both humans and animals, BA is excreted unchanged in the urine regardless of the route of administration. It is rapidly excreted, with a half-life of < 24 h in humans and animals. BA is slowly eliminated from bone.
In humans, 99% of a single iv dose of BA was excreted in the urine, and the half-life was estimated to be 21 h, based on a three-compartment pharmacokinetic model[19]. In another study by the same investigators, 94% of an oral dose of BA (aqueous solution) was recovered in the urine of a group of male volunteers, and more than 50% of the oral dose was eliminated in the first 24 h, consistent with the 21-h half-life in the iv study[19].
Because of boric acid contains cumulative toxicity, FAO/WHO Expert Committee declared that boric acid is unsafe to use as food additives. Even though Ministry of Health Malaysia[29] does not allow boric acid to be used as a food additive, however, it has been reported in some of the local foods in Malaysia such as yellow noodle and fish ball[30]. Moreover, boric acid is harmful to human health if consumed in higher amount. However, due to unawareness of the risk of boric acid, it is continued to be used in the production of food especially noodles and some processed seafood such as fish ball[31]. Boric acid normally used for preservation of food products. It can cause to health problem if the food containing boric acid was ingested by human as boric acid and borates are toxic to cell. Hence, it is deleterious to health and its usage is not recommended[31]. For new-born baby, the possible lethal doses are in between 3-6 g, whereas 15-20 g total for adults[32]. The common symptoms from several incidents of boric acid poisoning included coughing, eye irritation, vomiting and oral irritation[33]. However, the toxicity mechanisms of boron compound remain unclear (Kot, 2009). According to Moseman (1994)[34], the usual amount of boron in urine, blood and soft tissues, normally in the range below 0.05 mg kg−1 and do not above 10 mg kg−1. Some boric acid poisoning cases reported that as high as 2 g kg−1 boric acid was found in liver tissue and brain[34, 35].
W. G. Woods, An introduction to boron: history, sources, uses and chemistry, Environ. Health Perspect. 102(7), 5--11 (1994).
D. J. Fort, Adverse reproductive and developmental effects in Xenopus from insufficient boron, Biol. Trace Element Res. (current vol).
C. D. Eckert, Essentiality of boron for vertebrate embryonic development in zebrafish and trout, Biol. Trace Element Res. (current vol.).
C. L. Keen, Effects of very low boron exposure on rat development, Biol. Trace Element Res. (current vol.).
F. H. Nielsen, The saga of boron in food: from a banished food preservative to a beneficial nutrient for humans, Curr. Topics Plant Biochem. Physiol. 10, 274 (1991).
C. J. Rainey, R. E. Christensen, L. A. Nyquist, P. L. Strong, and J. R. Coughlin, Boron daily intake from the American diet, FASEB J. 10, A785 (abstract no. 4536) (1996).
Di Renzo, F., G. Cappelletti, M.L. Broccia, E. Giavini and E. Menegola, 2007. Boric acid inhibits embryonic histone deacetylases: A suggested mechanism to explain boric acid-related teratogenicity. Toxicol. Applied Pharmacol., 220: 178-185.
Cox, C., 2004. Boric acid and borates. J. Pesticide Reform, 24: 10-15.
Woods, W.G., 1994. An introduction to boron: History, sources, uses and chemistry. Environ. Health Perspect., 102: 5-11. PMID: 7889881
United States Environmental Protection Agency, 1993. Boric Acid. United States Environmental Protection Agency. Washington, DC.
United States Environmental Protection Agency, 1996. Report of the food quality protection act (FQPA) tolerance reassessment eligibility decision (TRED) for boric acid/sodium borate salts. United States Environmental Protection Agency.
Habes, D., S. Morakchi, N. Aribi, J.P. Farine and N. Soltani, 2006. Boric acid toxicity to the German cockroach, Blattella germanica: Alterations in midgut structure and acetylcholinesterase and glutathione S-transferase activity. Pesticide Biochem. Physiol., 84: 17-24.
Cochran, D.G., 1995. Toxic effects of boric acid on the German cockroach. Cell. Mol. Life Sci., 51: 561-563.
Clausen, C.A. and V. Yang, 2007. Protecting wood from mould, decay and termites with multicomponent biocide systems. Int. Biodeteriorat. Biodegradat., 59: 20-24.
Arslan, M., M. Topaktas and E. Rencuzogullari, 2008. The effects of boric acid on sister chromatid exchanges and chromosome aberrations in cultured human lymphocytes. Cytotechnology, 56: 91-96.
Normah, A., K.A. Ku Hasnah and M.H. Zainab, 1984. Penyalahgunaan asid borik dalam makanan. Teknol. Makanan, 3: 54-56.
Yiu, P.H., J. See., A. Rajan and C.F.J. Bong. 2008. Boric acid levels in fresh noodles and fish ball. Am. J. Agric. Biol. Sci., 3: 476-481.
J. S. Schou, J. A. Jansen, and B. Aggerbeck, Human pharmacokinetics and safety of boric acid, Arch. Toxicol. 7, 232-235 (1984).
J. A. Jansen, J. S. Schou, and B. Aggerbeck, Gastrointestinal absorption and in vitro release of boric acid from water-emulsifying ointments, Food Chem. Toxicol. 22, 49-53 (1984).
C. Job, Absorption and excretion of orally administered boron, Z. Angew. Bader-und Klimaheilkunde 20, 137-142 (1973).
H. I. Maibach, In vivo percutaneous absorption of boric acid, borax, and disodium octaborate tetrahydrate in humans, Biol. Trace Element Res. (current vol.).
B. Friis-Hansen, B. Aggerbeck, and J. A. Jansen, Unaffected blood boron levels in newborn infants treated with a boric acid ointment, Food Chem. Toxicol. 20, 451-454 (1982).
K. H. Beyer, W. F. Bergfeld, W. O. Berndt, R. K. Boutewell, W. W. Carlton, D. K. Hoffman, et al., Final report on the safety assessment of sodium borate and boric acid, ]. Am. Coil. Toxicol. 2(7), 87-125 (1983).
G. Stuttgen, T. Siebel, and B. Aggerbeck, Absorption of boric acid through human skin depending on the type of vehicle, Arch. Dermatol. Res. 272, 21-29 (1982).
G. V. Alexander, R. E. Nusbaum, and N. S. MacDonald, The boron and lithium content of human bones, ]. Biol. Chem. 192, 489-496 (1951).
R. M. Forbes, A. R. Cooper, and H. H. Mitchell, On the occurrence of beryllium, boron, cobalt, and mercury in human tissues, J. Biol. Chem. 209, 857-864 (1954).
N. L. Ward, The determination of boron in biological materials by neutron irradiation and prompt gamma-ray spectrometry, J. Radioanalytical Nuclear Chem. 110(2), 633-639 (1987).
J. Emsley, The Elements, Clarendon, Oxford, p. 32 (1989)
MDC Legal Advisers, 2004. Food Act and Regulation: All Amendment up to April, 2004: Act 281. MDC Publisher Sdn. Bhd. Kuala Lumpur, ISBN: 967-700808-0, pp: 394.
Siti-Mizura, S., E.S. Tee and H.E. Ooi, 1991. Determination of boric acid in foods: Comparative study of three methods. J. Sci. Food Agric., 55: 261-268.
Yiu, P.H., J. See., A. Rajan and C.F.J. Bong. 2008. Boric acid levels in fresh noodles and fish ball. Am. J. Agric. Biol. Sci., 3: 476-481. 32.
Toxicity Litovitz, T.L., W. Klein-Schwartz, G.M. Oderda and B.F. Schmitz, 1988. Clinical manifestations of toxicity in a series of 784 boric acid ingestions.Am. J. Emerg. Med., 6: 209-213.
Baker, D.M. and S.C. Bogema, 1986. Ingestion of boric acid by infants. Am. J. Emergency Med., 4: 358-361.
Kot, F.S., 2009. Boron sources, speciation and its potential impact on health. Rev. Environ. Sci. Biotechnol., 8: 3-28.
Moseman, R.F., 1994. Chemical disposition of boron in animals and humans. Environ. Health Perspect., 102: 113-117.
Boric acid is used in producing the glass faceplates of LCD flat panel displays. In electroplating, boric acid is used as part of some proprietary formulas. It is also used in the manufacturing of “remming mass”, a fine silica-containing powder used for producing induction furnace linings. Borates including boric acid have been used since the time of the Greeks for cleaning, preserving food, and other activities. It is used in pyrotechnics to prevent the amide-forming reaction between aluminum and nitrates. A small amount of boric acid is added to the composition to neutralize alkaline amides that can react with the aluminum. Boric acid dissolved in methane is popularly used among fire jugglers and fire spinners to create a deep green flame. Boric acid is added to salt in the curing of cattle hides, calfskins and sheepskins. Used in that way it helps to control bacteria development and also aids in the control of insects.
Trioxoboric(III) acid is the full systematic name for the solid acid and it exists in this form in its dilute solutions. However, in more concentrated solutions polymerization occurs to give polydioxoboric(III) acid.
In the solid there is considerable hydrogen bonding between H3BO3 molecules resulting in a layer structure, which accounts for the easy cleavage of the crystals. H3BO3 molecules also exist in dilute solutions but in more concentrated solutions polymeric acids and ions are formed (e.g. H4B2O7; pyroboric acid or tetrahydroxomonoxodiboric(III) acid). The compound is a very weak acid but also acts as a Lewis acid in accepting hydroxide ions:
B(OH)3 + H2O→B(OH)4 - + H+
If solid boric acid is heated it loses water and transforms to another acid at 300℃. This is given the formula HBO2 but is in fact a polymer (HBO2)n. It is called metaboric acid or, technically, polydioxoboric(III) acid.
Boric acid is used in the manufacture of glass (borosilicate glass), glazes and enamels, leather, paper, adhesives, and explosives. It is widely used (particularly in the USA) in detergents, and because of the ability of fused boric acid to dissolve other metal oxides it is used as a flux in brazing and welding. Because of its mild antiseptic properties it is used in the pharmaceutical industry and as a food preservative.
Company Profile Introduction
Shijiazhuang Tongyang Import and Export Co., LTD , located in Shijiazhuang City,which is the capital of Hebei Province and hub sector among Beijing, Tianjin, and Hebei.We have the advantage of convenient transportation.Our company is a modern high-tech chemical enterprise with Research & Development, production and sales.In recent five years, the company has implemented the strategy of "going out" vigorously.We has established our branches in Hubei China, Vietnam and Mexico, making our market and sales network more and more perfect.Our company will continue to adhere to the strategic positioning of fine chemical industry in the future and competition ideas of irreplaceable product differentiation,and strive to become to the leading of Chinese chemical industry.Shijiazhuang Tongyang Import and Export Co., LTD , located in Shijiazhuang City,which is the capital of Hebei Province and hub sector among Beijing, Tianjin, and Hebei.We have the advantage of convenient transportation.Our company is a modern high-tech chemical enterprise with Research & Development, production and sales.In recent five years, the company has implemented the strategy of "going out" vigorously.We has established our branches in Hubei China, Vietnam and Mexico, making our market and sales network more and more perfect.Our company will continue to adhere to the strategic positioning of fine chemical industry in the future and competition ideas of irreplaceable product differentiation,and strive to become to the leading of Chinese chemical industry.
Recommended supplier
-
VIP1年
- S D Fine Chem Limited
- 10043-35-3 / 11113-50-1 99%
- Inquiry
- 2024-03-16
-
VIP1年
- Paras Chemical Industries
- Boric acid 99%
- Inquiry
- 2024-02-29
-
VIP1年
- Indo Borax and Chemicals Ltd
- 10043-35-3 / 11113-50-1 99%
- Inquiry
- 2024-02-24
-
VIP1年
- Gujarat Boron Derivatives Pvt Ltd
- 10043-35-3 / 11113-50-1 Boric acid 98%
- Inquiry
- 2024-02-24
-
VIP1年
- Innovacorp India Pvt Ltd
- Boric acid 10043-35-3 / 11113-50-1 99%
- Inquiry
- 2024-02-08
-
VIP1年
- Hemadri Chemicals
- 10043-35-3 / 11113-50-1 98%
- Inquiry
- 2024-02-08
-
VIP1年
- Expresolv Limited
- 10043-35-3 / 11113-50-1 Boric acid 98%
- Inquiry
- 2024-02-07
-
VIP1年
- Shriji Chemicals
- Boric acid 10043-35-3 / 11113-50-1 98%
- Inquiry
- 2024-02-06
-
VIP1年
- Nandu Chemical Industries
- Boric acid 99%
- Inquiry
- 2024-02-01
-
VIP1年
- Indenta Chemicals (India) Pvt Ltd
- 10043-35-3 / 11113-50-1 Boric acid 98%
- Inquiry
- 2024-01-19
- Since:2015-05-06
- Address: room 1916,haiyuetiandi,66 yuehua west road,xiaoxi district