





safe shipping Pregabalin 148553-50-8
| Price | USD200.00 |
| Packge | 1KG |
- Min. Order:1KG
- Supply Ability:50ton
- Time:2021-08-07
Product Details
- Product Name Pregabalin /Lyrica
- CAS No.148553-50-8
- EINECS No.200-002-5
- MFC8H17NO2
- MW159.23
- Appearancewhite powder
- Melting point 194-196°C
- storage temp. 2-8°C
- Boiling point 274.0±23.0 °C(Predicted)
- density 0.997±0.06 g/cm3(Predicted)
- Water Solubility Soluble to 100 mM in water
| Product Name: | Pregabalin |
| Synonyms: | 3(S)-(AMINOMETHYL)-5-METHYLHEXANOIC ACID;(3S)-3-(AMINOMETHYL)-5-METHYLHEXANOIC ACID;PREGABALIN;Pregablin;3-(Aminomethyl)-5-methyl-hexanoic acid;PREDNISOLONESODIUMPHOSPHATE;(R)-Pregabalin;(S)-Pregabalin |
| CAS: | 148553-50-8 |
| MF: | C8H17NO2 |
| MW: | 159.23 |
| EINECS: | 604-639-1 |
| Product Categories: | Miscellaneous Biochemicals;APIs;Intermediates & Fine Chemicals;Neurochemicals;Pharmaceuticals;API;Pfizer compounds;LYRICA;pharmaceutical |
| Mol File: | 148553-50-8.mol |
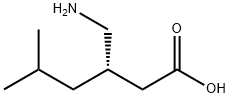 | |
It is usually unnecessary to ensure that patients are maintained on a specific manufacturer’s product unless there are specific concerns, such as patient anxiety and risk of confusion/ dosing error.
Adjunctive therapy of focal seizures with and without secondary generalization.
Recommendations summarized from NICE (2012)
Seizure types—on referral to tertiary care (focal seizures), contraindicated (generalized tonic- clonic seizures, tonic/ atonic seizures, absence seizures, myoclonic seizures).
Epilepsy types—on referral to tertiary care (benign epilepsy with centrotemporal spikes, panayiotopoulos syndrome, late- onset childhood occipital epilepsy), contraindicated (absence syndromes, idiopathic generalized epilepsy, juvenile myoclonic epilepsy, Dravet syndrome, Lennox– Gastaut syndrome).
Psychiatry
Generalized anxiety disorder.
Neurology
Peripheral and central neuropathic pain.
Epilepsy— adjunctive therapy: 25 mg bd for 7 days, to be increased by 50 mg every 7days; usual maintenance 300 mg daily, divided into 2 or 3 doses (max. 600 mg daily, divided into 2 or 3 doses).
Generalized anxiety disorder: 150 mg daily, divided into 2 or 3 doses, for 7 days, to be increased by 150 mg every 7 days (max. 600 mg daily, divided into 2 or 3 doses).
In December 2008, the US Food and Drug Administration (FDA) approved pregabalin (trade name "Lyrica") for the treatment of diabetic peripheral neuropathic pain (DPN) and postherpetic neuralgia (PHN)which are Both the most common neuropathic pains.
Neuropathic pain is one of the most difficult chronic pain syndromes to treat , dull pain, burning, tingling as the main feature, there are a lot of incentives of neuralgia, diabetes, infections (such as herpes zoster), cancer and AIDS, etc. can cause neurological pain, in Europe about 3% of the population suffer from neuralgia torture.
The above information is edited by the chemicalbook of Tian Ye.
Patients with conditions that may precipitate encephalopathy.
Patients with severe congestive heart failure.
Since pregabalin is predominantly excreted unchanged in the urine, undergoes negligible metabolism in humans, does not inhibit drug metabolism in vitro, and is not bound to plasma proteins, it is unlikely to produce or be subject to pharmacokinetic interactions.
Pregabalin may potentiate the effects of lorazepam.
In the post- marketing experience, there are reports of respiratory failure and coma in patients taking pregabalin and other central nervous system depressant medicinal products. Pregabalin appears to be additive in the impairment of cognitive and gross motor function caused by oxycodone.
There are no specific foods that must be excluded from diet when taking pregabalin.
Pregabalin may potentiate the effects of alcohol.
No dose adjustment is required for patients with hepatic impairment.
Renal impairment
Reduce maintenance dose according to degree of reduction in creatinine clearance.
Pregnancy
There is no adequate data from the use of pregabalin in pregnant women. The potential risk for reproductive toxicity in humans is unknown. Pregabalin should not be used during pregnancy unless the benefit to the mother clearly outweighs the potential risk to the foetus.
Pregabalin is excreted into human milk. The effect of pregabalin on newborns/ infants is unknown. A case- by- case decision must be made whether to discontinue breast- feeding or to discontinue pregabalin therapy taking into account the benefit of breastfeeding for the child and the benefit of therapy for the woman.
Company Profile Introduction
Shijiazhuang Tongyang Import and Export Co., LTD , located in Shijiazhuang City,which is the capital of Hebei Province and hub sector among Beijing, Tianjin, and Hebei.We have the advantage of convenient transportation.Our company is a modern high-tech chemical enterprise with Research & Development, production and sales.In recent five years, the company has implemented the strategy of "going out" vigorously.We has established our branches in Hubei China, Vietnam and Mexico, making our market and sales network more and more perfect.Our company will continue to adhere to the strategic positioning of fine chemical industry in the future and competition ideas of irreplaceable product differentiation,and strive to become to the leading of Chinese chemical industry.Shijiazhuang Tongyang Import and Export Co., LTD , located in Shijiazhuang City,which is the capital of Hebei Province and hub sector among Beijing, Tianjin, and Hebei.We have the advantage of convenient transportation.Our company is a modern high-tech chemical enterprise with Research & Development, production and sales.In recent five years, the company has implemented the strategy of "going out" vigorously.We has established our branches in Hubei China, Vietnam and Mexico, making our market and sales network more and more perfect.Our company will continue to adhere to the strategic positioning of fine chemical industry in the future and competition ideas of irreplaceable product differentiation,and strive to become to the leading of Chinese chemical industry.
Recommended supplier
-
VIP1年
- SUPREME DRUGS PVT LTD
- Pregabalin IP/USP 98%
- Inquiry
- 2024-09-19
-
VIP1年
- Vepan Pharmatech Pvt Ltd
- Pregabalin
- Inquiry
- 2024-09-19
-
VIP1年
- Blue Jet Healthcare Ltd
- Pregabalin 148553-50-8 >98%
- Inquiry
- 2024-07-06
-
VIP1年
- AKASH PHARMA EXPORTS
- Pregabalin 99%
- Inquiry
- 2024-05-16
-
VIP1年
- GN PAL Speciality Molecules LLP
- Pregabalin 148553-50-8 98%
- Inquiry
- 2024-05-15
-
VIP1年
- ADAARSH PHARMA
- Pragabalin -IP/BP 99%
- Inquiry
- 2024-05-03
-
VIP0年
- Ebenezer Industries
- 148553-50-8 Pregabalin 98%
- Inquiry
- 2024-03-30
-
VIP1年
- Adrhim Pharmaceutical LLP
- 148553-50-8 Pregabalin 99%
- Inquiry
- 2024-03-29
-
VIP1年
- Heer Pharma Pvt Ltd
- Pregabalin 148553-50-8 99%
- Inquiry
- 2024-03-23
-
VIP1年
- Enaltec Labs Pvt Ltd
- 148553-50-8 99%
- Inquiry
- 2024-03-21
- Since:2015-05-06
- Address: room 1916,haiyuetiandi,66 yuehua west road,xiaoxi district
