80-15-9
Product Name:
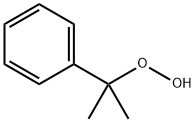
Cumyl hydroperoxide
Formula:
C9H12O2
Synonyms:
α,α-Dimethylbenzyl hydroperoxide
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Cumene hydroperoxide is a colorless to light yellow liquid with a sharp, irritating odor. Flash point 175 °F. Boils at 153 °C and at 100 °C at the reduced pressure of 8 mmHg. Slightly soluble in water and denser than water. Hence sinks in water. Readily soluble in alcohol, acetone, esters, hydrocarbons, chlorinated hydrocarbons. Toxic by inhalation and skin absorption. Used in production of acetone and phenol, as a polymerization catalyst, in redox systems. |
|---|---|
| Color/Form | Colorless to pale-yellow liq |
| Boiling Point | Decomposes at 261 °F (NTP, 1992) |
| Melting Point | less than -40 °F (NTP, 1992) |
| Flash Point | 135 °F (NTP, 1992) |
| Solubility | less than 0.1 mg/mL at 64 °F (NTP, 1992) |
| Density | 1.03 at 77 °F (USCG, 1999) - Denser than water; will sink |
| Vapor Density | 5 (NTP, 1992) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure | 0.6 mmHg at 68 °F (for 80-85% by weight) (NTP, 1992) |
| LogP | 2.16 |
| Autoignition Temperature | 300 °F (USCG, 1999) |
| Decomposition | At concentrations of 91 and 95%, cumene hydroperoxide decomposed violently at about 150 °C. |
| Corrosivity | Reactive with metal-containing materials. |
| Heat of Combustion | -7,400 cal/g= -310X10+5 J/kg |
| Heat of Vaporization | 6.99X10+7 J/kmol at 298.15 K |
| Surface Tension | 2.8X10-2 N/m at 264 K |
| Refractive Index | Index of refraction: 1.5242 at 20 °C |
| Other Experimental Properties | Active oxygen content 10.5% |
| Chemical Classes | Plastics & Rubber -> Curing Agents (Aromatic) |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H242:Self-reactive substances and mixtures; and Organic peroxides H304:Aspiration hazard H314:Skin corrosion/irritation H331:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H373:Specific target organ toxicity, repeated exposure H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P370+P378:In case of fire: Use … for extinction. |
COMPUTED DESCRIPTORS
| Molecular Weight | 152.19 g/mol |
|---|---|
| XLogP3 | 1.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 152.083729621 g/mol |
| Monoisotopic Mass | 152.083729621 g/mol |
| Topological Polar Surface Area | 29.5 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 115 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Cumene hydroperoxide is a colorless to light yellow liquid with a sharp, irritating odor. Flash point 175 °F. Boils at 153 °C and at 100 °C at the reduced pressure of 8 mmHg. Slightly soluble in water and denser than water. Hence sinks in water. Readily soluble in alcohol, acetone, esters, hydrocarbons, chlorinated hydrocarbons. Toxic by inhalation and skin absorption. Used in production of acetone and phenol, as a polymerization catalyst, in redox systems.