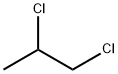
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | 1,2-dichloropropane is a colorless watery liquid with a sweet odor. Sinks in water. Produces an irritating vapor. (USCG, 1999) |
|---|---|
| Color/Form | Colorless liquid |
| Odor | Sweet |
| Boiling Point | 203 to 205 °F at 760 mmHg (NTP, 1992) |
| Melting Point | -148 °F (NTP, 1992) |
| Flash Point | 60 °F (NTP, 1992) |
| Solubility | less than 0.1 mg/mL at 70.7 °F (NTP, 1992) |
| Density | 1.158 at 68 °F (USCG, 1999) - Denser than water; will sink |
| Vapor Density | 3.9 (NTP, 1992) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure | 40 mmHg at 66.9 °F ; 42 mmHg at 68 °F (NTP, 1992) |
| LogP | log Kow = 1.98 |
| Henry's Law Constant | Henry's Law constant = 2.82X10-3 atm-cu m/mole @ 25 °C |
| Stability/Shelf Life | Sensitive to heat. |
| Autoignition Temperature | 1035 °F (USCG, 1999) |
| Decomposition | When heated to decomposition it emits toxic fumes of /hydrogen chloride/. |
| Corrosivity | ... It will attack some forms of plastics, rubber, & coatings. |
| Heat of Vaporization | 8,428.5 g cal/g mole (multiply by 4.184 for J/g mole) |
| Surface Tension | 29 dynes/cm = 0.029 N/m at 20 °C |
| Ionization Potential | 10.87 eV |
| Odor Threshold | Odor Threshold Low: 0.26 [mmHg] Odor Threshold High: 0.52 [mmHg] Detection odor threshold from AIHA (mean = 0.26 ppm) |
| Refractive Index | Index of refraction: 1.4388 at 20 °C/D |
| Relative Evaporation Rate | Greater than 1 (butyl acetate= 1) |
| Kovats Retention Index | 672 688 677.6 688 668.9 688 684 675.7 678.6 691 684 691 691 670 674 684 678.9 667 679 |
| Other Experimental Properties | Wt/gal: 9.6 lb @ 20 °C |
| Chemical Classes | Solvents -> Chlorinated Aliphatics |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H302:Acute toxicity,oral H331:Acute toxicity,inhalation H350:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P308+P313:IF exposed or concerned: Get medical advice/attention. |
COMPUTED DESCRIPTORS
| Molecular Weight | 112.98 g/mol |
|---|---|
| XLogP3 | 1.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 1 |
| Exact Mass | 111.9846556 g/mol |
| Monoisotopic Mass | 111.9846556 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 20.9 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
1,2-Dichloropropane is a colorless, flammable liquid with a chloroform-like odor. It is moderately soluble in water and readily evaporates into air. It does not occur naturally in the environment. 1,2-Dichloropropane production in the United States has declined over the past 20 years. It was used in the past as a soil fumigant, chemical intermediate and industrial solvent and was found in paint strippers, varnishes, and furniture finish removers. Most of these uses were discontinued. Today, almost all of the 1,2-dichloropropane is used as a chemical intermediate to make perchloroethylene and several other related chlorinated chemicals.