75-71-8
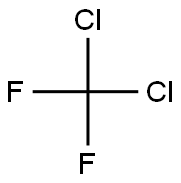
Product Name:
Dichlorodifluoromethane
Formula:
CCl2F2
Synonyms:
Anti-DKFZp686M18273;Anti-G protein-coupled receptor 17;G protein-coupled receptor 17
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Dichlorodifluoromethane appears as a colorless gas having a faint ethereal odor. Shipped as a liquid confined under its own vapor pressure. Contact with the unconfined liquid can cause frostbite. Both components are noncombustible. Can asphyxiate by the displacement of air. Exposure of the closed container to prolonged heat or fire can cause it to rupture violently and rocket. |
|---|---|
| Color/Form | Colorless gas ... [Note: Shipped as a liquified compressed gas] |
| Odor | Practically odorless ... faint, ether-like odor in high concentration |
| Boiling Point | -21.6 °F at 760 mmHg (NTP, 1992) |
| Melting Point | -252 °F (NTP, 1992) |
| Solubility | Insoluble (NTP, 1992) |
| Density | 1.35 at 59 °F (USCG, 1999) - Denser than water; will sink |
| Vapor Density | 4.2 (NIOSH, 2023) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure | 5 atm at 61 °F (NTP, 1992) |
| LogP | log Kow = 2.16 |
| Henry's Law Constant | Henry's Law constant = 0.343 atm-cu m/mol at 20 °C |
| Stability/Shelf Life | Stable up to 550 °C. |
| Autoignition Temperature | Not flammable (USCG, 1999) |
| Decomposition | When heated to decomp it emits highly toxic fumes of phosgene and /hydrogen chloride and hydrogen fluoride/. |
| Viscosity | 0.262 centipoise at 70 °F |
| Corrosivity | Noncorrosive |
| Heat of Combustion | Nonflammable |
| Heat of Vaporization | 20.1 kJ/mol at -29.8 °C |
| Surface Tension | 9 dynes/cm |
| Ionization Potential | 11.75 eV |
| Kovats Retention Index | 318 314 319.2 311 299.9 313 305 313 305 |
| Other Experimental Properties | Dipole moment: 0.51 debye. Absorbs less than 0.0025% water. |
| Chemical Classes | Solvents -> Chlorofluorocarbons |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H370:Specific target organ toxicity, single exposure H420:Hazardous to the ozone layer |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P311:Call a POISON CENTER or doctor/physician. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
COMPUTED DESCRIPTORS
| Molecular Weight | 120.91 g/mol |
|---|---|
| XLogP3 | 2.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 119.9345117 g/mol |
| Monoisotopic Mass | 119.9345117 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 30.6 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Dichlorodifluoromethane appears as a colorless gas having a faint ethereal odor. Shipped as a liquid confined under its own vapor pressure. Contact with the unconfined liquid can cause frostbite. Both components are noncombustible. Can asphyxiate by the displacement of air. Exposure of the closed container to prolonged heat or fire can cause it to rupture violently and rocket.