75-69-4
Product Name:
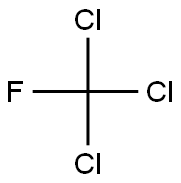
Trichlorofluoromethane
Formula:
CCl3F
Synonyms:
CFC-11;Fluorotrichloromethane;Freon 11
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Trichlorofluoromethane appears as a clear light colored liquid. Nearly odorless. Denser than water. Poses low acute health hazard to humans. Primary hazard is to the environment. Immediate steps should be taken to limit spread to the environment. Easily penetrates the soil to contaminate groundwater and nearby waterways. |
|---|---|
| Color/Form | Volatile liquid or gas |
| Odor | In concentration of less than 20% (by volume in air), trichlorofluoromethane is odorless, in higher concentration, its odor is mild and somewhat ethereal. |
| Boiling Point | 74.7 °F at 760 mmHg (NTP, 1992) |
| Melting Point | -168 °F (NTP, 1992) |
| Solubility | 10 to 50 mg/mL at 64 °F (NTP, 1992) |
| Density | 1.49 at 63 °F (NTP, 1992) - Denser than water; will sink |
| Vapor Density | 5.04 at 77 °F (NTP, 1992) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure | 792 mmHg at 77 °F (NTP, 1992) |
| LogP | log Kow = 2.53 |
| Henry's Law Constant | Henry's Law constant = 9.70X10-2 atm-cu cm/mol at 25 °C |
| Stability/Shelf Life | The product is normally stable. |
| Autoignition Temperature | Not flammable (USCG, 1999) |
| Decomposition | Decomposition products include hydrochloric acid, hydrofluoric acid, and carbonyl halides. |
| Viscosity | Gas: 0.0105 mPa.s at 24 °C; liquid: 0.43 mPa.s at 20 °C |
| Corrosivity | Liquid fluorotrichloromethane will attack some forms of plastics, rubber, and coatings. |
| Heat of Vaporization | 25.1 kJ/mol at 23.7 °C |
| Surface Tension | 18 dyn/cm at 25 °C |
| Ionization Potential | 11.77 eV |
| Odor Threshold | Odor Threshold Low: 200000.0 [mmHg] Odor threshold is 200,000 ppm (20% by volume). [CHEMINFO] VP from HSDB |
| Refractive Index | Index of refraction: 1.3865 @ 18.5 °C/D |
| Relative Evaporation Rate | 63 (Butyl acetate = 1) |
| Kovats Retention Index | 470 486 486 482 474.9 478 478 484 475.9 484 |
| Other Experimental Properties | Global warming potential: 1.0; ozone depletion potential: 1.0 |
| Chemical Classes | Solvents -> Chlorofluorocarbons |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H331:Acute toxicity,inhalation H336:Specific target organ toxicity,single exposure; Narcotic effects H351:Carcinogenicity H372:Specific target organ toxicity, repeated exposure H420:Hazardous to the ozone layer |
| Precautionary Statement Codes |
P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. P502:Refer to manufacturer/supplier for information on recovery/recycling |
COMPUTED DESCRIPTORS
| Molecular Weight | 137.36 g/mol |
|---|---|
| XLogP3 | 2.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 135.904961 g/mol |
| Monoisotopic Mass | 135.904961 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 28.4 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Trichlorofluoromethane appears as a clear light colored liquid. Nearly odorless. Denser than water. Poses low acute health hazard to humans. Primary hazard is to the environment. Immediate steps should be taken to limit spread to the environment. Easily penetrates the soil to contaminate groundwater and nearby waterways.