7487-94-7
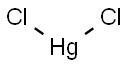
Product Name:
Mercury chloride
Formula:
Cl2Hg
Synonyms:
Mercuric chloride;Mercury dichloride;Mercury(II) chloride
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Mercuric chloride appears as an odorless white crystalline solid. Density 5.4 g / cm3. Melting point 277 °C. Slightly volatile at ordinary temperatures. Can be sublimed unchanged. Corrosive to the mucous membranes. Toxic by inhalation (dusts, etc.), ingestion, and skin absorption. Used in photography, disinfectants, wood preservatives, fungicides. |
|---|---|
| Color/Form | Colorless rhombic crystals or white powder |
| Odor | Odorless |
| Boiling Point | 576 °F at 760 mmHg (EPA, 1998) |
| Melting Point | 529 °F (EPA, 1998) |
| Solubility | 5 to 10 mg/mL at 72 °F (NTP, 1992) |
| Density | 5.44 at 77 °F (EPA, 1998) - Denser than water; will sink |
| Vapor Density | 9.8 g/cu cm |
| Vapor Pressure | 1 mmHg at 277.16 °F (EPA, 1998) |
| LogP | log Kow= 0.22 |
| Henry's Law Constant | Henry's Law constant = 7.09X10-10 atm-cu m/mole 25 °C |
| Stability/Shelf Life | SLIGHTLY VOLATILE AT ORDINARY TEMP; APPRECIABLY SO AT 100 °C; VOLATILIZES UNCHANGED @ ABOUT 300 °C |
| Corrosivity | Corrosion rate: 0.0003 mm/yr (1% concn) at 100 °C; 0.01 mm/yr (5% concn) at 100 °C; 0.001 mm/yr (10% concn) at 100 °C. ... relative to titanium |
| pH | About 4.7 also reported as 3.2 for 0.2 molar aq soln |
| Refractive Index | INDEX OF REFRACTION: 1.859 |
| Other Experimental Properties | Coagulates albumin; produces yellow precipitate with sodium hydroxide |
| Chemical Classes | Metals -> Mercury Compounds, Inorganic |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H300:Acute toxicity,oral H314:Skin corrosion/irritation H341:Germ cell mutagenicity H372:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 271.50 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 0 |
| Exact Mass | 271.908349 g/mol |
| Monoisotopic Mass | 271.908349 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 3 |
| Formal Charge | 0 |
| Complexity | 2.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Mercuric chloride appears as an odorless white crystalline solid. Density 5.4 g / cm3. Melting point 277 °C. Slightly volatile at ordinary temperatures. Can be sublimed unchanged. Corrosive to the mucous membranes. Toxic by inhalation (dusts, etc.), ingestion, and skin absorption. Used in photography, disinfectants, wood preservatives, fungicides.