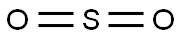CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Sulfur dioxide appears as a colorless gas with a choking or suffocating odor. Boiling point -10 °C. Heavier than air. Very toxic by inhalation and may irritate the eyes and mucous membranes. Under prolonged exposure to fire or heat the containers may rupture violently and rocket. Used to manufacture chemicals, in paper pulping, in metal and food processing. Rate of onset: Immediate & Delayed Persistence: Minutes to hours Odor threshold: 1 ppm Source/use/other hazard: Disinfectant and preserving in breweries and food/canning; textile industry; batteries. |
|---|---|
| Color/Form | Colorless gas or liquid |
| Odor | Strong suffocating odor |
| Taste | Acid taste |
| Boiling Point | 14 °F at 760 mmHg (EPA, 1998) |
| Melting Point | -98.9 °F (EPA, 1998) |
| Solubility | 10 % (NIOSH, 2023) |
| Density | 1.434 (EPA, 1998) - Denser than water; will sink |
| Vapor Density | 2.26 (EPA, 1998) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure | 2432 mmHg at 68 °F (EPA, 1998) |
| Henry's Law Constant | Henry's Law constant = 8.10X10-4 atm-cu m/mol at 25 °C |
| Stability/Shelf Life | Stable under recommended storage conditions. |
| Autoignition Temperature | Not flammable (USCG, 1999) |
| Viscosity | Gas: 0.0124 mPa.s at 18 °C. Liquid: 0.368 mPa.s at 0 °C. |
| Corrosivity | Iron, steel, nickel, copper-nickel alloys, & inconel nickel-chromium-iron are satisfactory for dry or hot sulfur dioxide, but they are readily corroded below the dew point or by wet sulfur dioxide gas. Liquid sulfur dioxide produces serious corrosion of iron, brass, and copper at about 0.2 wt% or higher moisture content. |
| Heat of Vaporization | 22.92 kJ/mol |
| Surface Tension | 28.59 mN/m (liquid at 10 °C) |
| Ionization Potential | 12.30 eV |
| Odor Threshold | Odor Threshold Low: 0.33 [ppm] Odor Threshold High: 5.0 [ppm] Detection odor threshold from AIHA (mean = 2.7) |
| Refractive Index | Index of refraction: 1.3396 at 25 °C |
| Relative Evaporation Rate | Greater than 1 (Butyl acetate = 1) |
| Kovats Retention Index | 856 882 |
| Other Experimental Properties | Heat of formation: -296.8 kJ/mol at 298.15 K; standard molar entropy: 248.2 J/K-mol at 298.15 K; heat capacity: 39.9 J/mol-K at constant pressure at 298.15 K /gas/ |
| Chemical Classes | Toxic Gases & Vapors -> Corrosive Gases |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Gas Cylinder Compressed Gases GHS04  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H280:Gases under pressure H314:Skin corrosion/irritation H331:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P410+P403:Protect from sunlight. Store in a well-ventilated place. |
COMPUTED DESCRIPTORS
| Molecular Weight | 64.07 g/mol |
|---|---|
| XLogP3 | 0.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 63.96190041 g/mol |
| Monoisotopic Mass | 63.96190041 g/mol |
| Topological Polar Surface Area | 35.1 Ų |
| Heavy Atom Count | 3 |
| Formal Charge | 0 |
| Complexity | 18.3 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Sulfur dioxide is a colorless gas with a pungent odor. It is a liquid when under pressure, and it dissolves in water very easily. Sulfur dioxide in the air comes mainly from activities such as the burning of coal and oil at power plants or from copper smelting. In nature, sulfur dioxide can be released to the air from volcanic eruptions.