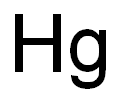CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Mercury appears as an odorless, silvery metallic liquid. Insoluble in water. Toxic by ingestion, absorption and inhalation of the fumes. Corrosive to aluminum. Used as a catalyst in instruments, boilers, mirror coatings. |
|---|---|
| Color/Form | Silver-white, heavy, mobile, liquid metal; solid mercury is tin-white |
| Odor | Odorless |
| Boiling Point | 675 °F at 760 mmHg (USCG, 1999) |
| Melting Point | -38 °F (USCG, 1999) |
| Solubility | Insoluble (NIOSH, 2023) |
| Density | 13.55 at 68 °F (USCG, 1999) - Denser than water; will sink |
| Vapor Density | Relative vapor density (air = 1): 6.93 |
| Vapor Pressure | 0.0012 mmHg (NIOSH, 2023) |
| Stability/Shelf Life | SLIGHTLY VOLATILE AT ORDINARY TEMP; WHEN PURE, DOES NOT TARNISH ON EXPOSURE TO AIR AT ORDINARY TEMP, BUT WHEN HEATED TO NEAR BOILING POINT, SLOWLY OXIDIZES TO MERCURIC OXIDE (HGO) |
| Autoignition Temperature | Not flammable (USCG, 1999) |
| Viscosity | 1.55 mPa.sec (15.5 millipoise) at 20 °C |
| Corrosivity | The high mobility and tendency to dispersion exhibited by mercury, and the ease with which it forms alloys (amalga) with many laboratory and electrical contact metals, can cause severe corrosion problems in laboratories. |
| Heat of Vaporization | 14.652 kcal/mole @ 25 °C |
| Surface Tension | 470 dynes/cm @ 20 °C |
| Refractive Index | Index of refraction: 1.6 to 1.9 @ 20 °C |
| Other Experimental Properties | Ductile malleable mass which may be cut with a knife; atomic number 80; valences 1 and 2; group 2B element of periodic table; natural isotopes 202 (29.80%), 200 (23.13%), 199 (16.84%), 201 (13.22%), 198 (10.02%), 204 (6.85%) and 196 (0.146%); electrical resistivity 95.76 microohm cm at 20 °C; forms alloys with most metals except iron and combines with sulfur at ordinary temp; reacts with HNO3, hot concn H2SO4, and ammonia solutions to form Hg2NOH (Millon's base); std electrode reduction potential: eo (aq) Hg/Hg2+ equals -0.854 volts; eo (aq) 2 Hg/2Hg2+ equals -0.789 volts |
| Chemical Classes | Metals -> Elements, Metallic |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H330:Acute toxicity,inhalation H372:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P273:Avoid release to the environment. |
COMPUTED DESCRIPTORS
| Molecular Weight | 200.59 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 0 |
| Exact Mass | 201.970644 g/mol |
| Monoisotopic Mass | 201.970644 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 1 |
| Formal Charge | 0 |
| Complexity | 0 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Mercurycombines with other elements, such as chlorine, sulfur, or oxygen, to form inorganic mercury compounds or "salts", which are usually white powders or crystals. Mercury also combines with carbon to make organic mercury compounds. The most common one, methylmercury, is produced mainly by microscopic organisms in the water and soil. More mercury in the environment can increase the amounts of methylmercury that these small organisms make.Metallic Mercuryis a dense liquid that vaporizes easily at room temperature. Metallic mercury is not easily absorbed into unbroken skin. However, it vaporizes, even at room temperature. The higher the temperature, the more vapors are released. Mercury vapors are colorless and odorless, though they can be seen with the aid of an ultraviolet light.Metallic mercury is used to produce chlorine gas and caustic soda, and is also used in thermometers, dental fillings, and batteries. Mercury salts are sometimes used in skin lightening creams and as antiseptic creams and ointments.