7783-35-9
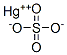
Product Name:
MERCURY(II) SULFATE
Formula:
HgO4S
Synonyms:
Mercuric sulfate;Mercury(II) sulfate
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Mercuric sulfate appears as odorless white granules or crystalline powder. Denser than water. It is toxic by inhalation and by ingestion. It is used in medicine, for gold and silver extraction, and to make other mercury compounds. |
|---|---|
| Color/Form | White granules or crystalline powder |
| Odor | Odorless |
| Solubility | Sol in hydrochloric acid, hot dil sulfuric acid, concentrated sodium chloride |
| Density | 6.47 at 68 °F (USCG, 1999) - Denser than water; will sink |
| Decomposition | 450 °C. This produces very toxic fumes of mercury and sulfur oxides. The solution in water is a medium strong acid. Reacts with hydrogen halides. Attacks metal in the presence of moisture. |
| Corrosivity | Corrosiveness: Magnesium; Aluminum; Zinc; Iron; Lead; Copper |
| Other Experimental Properties | Decomp by water into yellow insol, basic sulfate, and free sulfuric acid. |
| Chemical Classes | Metals -> Mercury Compounds, Inorganic |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H373:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P262:Do not get in eyes, on skin, or on clothing. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P314:Get medical advice/attention if you feel unwell. |
COMPUTED DESCRIPTORS
| Molecular Weight | 296.66 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 297.922373 g/mol |
| Monoisotopic Mass | 297.922373 g/mol |
| Topological Polar Surface Area | 88.6 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 62.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Mercuric sulfate appears as odorless white granules or crystalline powder. Denser than water. It is toxic by inhalation and by ingestion. It is used in medicine, for gold and silver extraction, and to make other mercury compounds.
