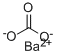CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Barium carbonate is a white powder. It is insoluble in water and soluble in most acids, with the exception of sulfuric acid. It has a specific gravity of 4.275. It is toxic by ingestion. |
|---|---|
| Color/Form | White, heavy powder |
| Odor | Odorless |
| Taste | Tasteless |
| Melting Point | ERG is for "Barium compound, n.o.s." Vapor pressure is negligible. 811 °C |
| Solubility | In water, 0.0014 g/100 g at 20 °C |
| Density | 4.3 at 68 °F (USCG, 1999) - Denser than water; will sink |
| Vapor Pressure | Essentially zero |
| LogP | -1.32 (calculated) |
| Stability/Shelf Life | Thermally stable |
| Autoignition Temperature | Not flammable (USCG, 1999) |
| Refractive Index | Indices of refraction: 1.529, 1.676, 1.677 |
| Other Experimental Properties | At about 1300 °C, decomposes into barium oxide and carbon dioxide |
| Chemical Classes | Metals -> Metals, Inorganic Compounds |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
COMPUTED DESCRIPTORS
| Molecular Weight | 197.34 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 197.889991 g/mol |
| Monoisotopic Mass | 197.889991 g/mol |
| Topological Polar Surface Area | 63.2 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 18.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Barium carbonate is a white powder. It is insoluble in water and soluble in most acids, with the exception of sulfuric acid. It has a specific gravity of 4.275. It is toxic by ingestion.