2867-47-2
Product Name:
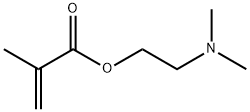
2-(Dimethylamino)ethyl methacrylate
Formula:
C8H15NO2
Synonyms:
(2-Dimethylaminoethyl) methacrylate;2-Propenoic acid-2-methyl-(2-dimethylamino)ethylester;Methacrylic acid 2-(dimethylamino)ethyl ester
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | 2-dimethylaminoethyl methacrylate appears as a clear colorless liquid. Less dense than water and insoluble in water. Vapors heavier than air and corrosive to eyes and mucous membranes. May polymerize exothermically if heated or contaminated. If polymerization takes place inside a closed container, the container may rupture violently. Produces toxic oxides of nitrogen during combustion. Toxic by skin absorption, ingestion and inhalation. Used to make plastics and in textiles. |
|---|---|
| Color/Form | Liquid |
| Odor | Amine-like |
| Boiling Point | 186 °C |
| Melting Point | -30 °C (freezing point) |
| Flash Point | 165 °F (NFPA, 2010) |
| Solubility | In water, 1000 g/L at 20 °C (study performed without adjustment of pH value) |
| Density | Specific gravity: 0.933 at 25 °C |
| Vapor Density | 5.4 (AIR= 1) |
| Vapor Pressure | 0.82 [mmHg] |
| LogP | log Kow = 1.13 at 25 °C; OECD Guideline 107 (Partition Coefficient (n-octanol/water), Shake Flask Method) |
| Autoignition Temperature | 255 °C |
| Decomposition | When heated to decomposition it emits toxic fumes of nitroxides. |
| Viscosity | 1.34 mPa s at 25 °C |
| Heat of Vaporization | 48.8 kJ/mol |
| Polymerization | METHYL METHACRYLATE, AND IN GENERAL THE METHACRYLIC ESTERS, POLYMERIZE MUCH LESS READILY THAN THE CORRESPONDING ORDINARY ACRYLATES. NONE THE LESS, THEY ARE STABILIZED BY ADDING HYDROQUINONE OR PYROGALLOL, PARTICULARLY IN THE PRESENCE OF METALLIC COPPER. /METHACRYLATES/ |
| Refractive Index | Index of refraction: 1.4396 at 20 °C |
| Dissociation Constants | pKa = 8.44 at 25 °C |
| Other Experimental Properties | Most aliphatic amines have an unpleasant, fishy or fishlike odor and in high concentrations they all have the odor of ammonia. /Aliphatic amines/ |
| Chemical Classes | Plastics & Rubber -> (Meth)acrylates |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 157.21 g/mol |
|---|---|
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 5 |
| Exact Mass | 157.110278721 g/mol |
| Monoisotopic Mass | 157.110278721 g/mol |
| Topological Polar Surface Area | 29.5 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 152 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
2-dimethylaminoethyl methacrylate appears as a clear colorless liquid. Less dense than water and insoluble in water. Vapors heavier than air and corrosive to eyes and mucous membranes. May polymerize exothermically if heated or contaminated. If polymerization takes place inside a closed container, the container may rupture violently. Produces toxic oxides of nitrogen during combustion. Toxic by skin absorption, ingestion and inhalation. Used to make plastics and in textiles.