181695-72-7
Product Name:
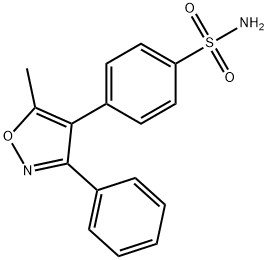
Valdecoxib
Formula:
C16H14N2O3S
Synonyms:
4-(5-Methyl-3-phenyl-4-isoxazolyl)benzenesulfonamide
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Color/Form | White crystalline powder |
| Melting Point | 160 - 162 °C |
| Solubility | >47.2 [ug/mL] (The mean of the results at pH 7.4) |
| Vapor Pressure | 4.6X10-10 mm Hg at 25 °C /Estimated/ |
| LogP | 3.2 |
| Henry's Law Constant | Henry's Law constant = 2.2X10-11 atm-cu m/mol at 25 °C /Estimated/ |
| Other Experimental Properties | Hydroxyl radical reaction rate constant = 1.3X10-11 cu cm/molec-sec at 25 °C /Estimated/ |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H373:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. |
COMPUTED DESCRIPTORS
| Molecular Weight | 314.4 g/mol |
|---|---|
| XLogP3 | 2.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 3 |
| Exact Mass | 314.07251349 g/mol |
| Monoisotopic Mass | 314.07251349 g/mol |
| Topological Polar Surface Area | 94.6 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 462 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Valdecoxib is a member of the class of isoxazoles that is isoxazole which is substituted at positions 3, 4 and 5 by phenyl, p-sulfamoylphenyl and methyl groups, respectively. A selective cyclooxygenase 2-inhibitor, it used as a nonsteroidal anti-inflammatory drug (NSAID) for the treatment of arthritis from 2001 until 2005, when it was withdrawn following concerns of an associated increased risk of heart attack and stroke. It has a role as a non-steroidal anti-inflammatory drug, a cyclooxygenase 2 inhibitor, a non-narcotic analgesic, an antirheumatic drug and an antipyretic. It is a member of isoxazoles and a sulfonamide.