144-62-7
Product Name:
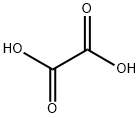
Oxalic acid
Formula:
C2H2O4
Synonyms:
Ethanedioic acid dihydrate;Oxalic acid;Oxalic acid solution
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Oxalic acid is an odorless white solid. Sinks and mixes with water. (USCG, 1999) |
|---|---|
| Color/Form | ANHYDROUS OXALIC ACID, CRYSTALLIZED FROM GLACIAL ACETIC ACID IS ORTHORHOMBIC, CRYSTALS BEING PYRAMIDAL OR ELONGATED OCTAHEDRA |
| Odor | Odorless. |
| Boiling Point | Sublimes (NIOSH, 2023) |
| Melting Point | 372 °F (Decomposes) (NTP, 1992) |
| Solubility | 50 to 100 mg/mL at 75 °F (NTP, 1992) |
| Density | 1.9 at 59 °F (USCG, 1999) - Denser than water; will sink |
| Vapor Density | 4.3 (NTP, 1992) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure | 0.001 mmHg at 68 °F (NTP, 1992) |
| LogP | -0.81 |
| LogS | 0.38 |
| Henry's Law Constant | Henry's Law constant: 1.4X10-10 atm-cu m/mole at 25 °C (experimental) |
| Stability/Shelf Life | OXALIC ACID CAN BE DEHYDRATED BY CAREFUL DRYING @ 100 °C, BUT LOSSES OCCUR THROUGH SUBLIMATION /OXALIC ACID DIHYDRATE/ |
| Autoignition Temperature | Not flammable (USCG, 1999) |
| Decomposition | ... DECOMP PRODUCTS INCL CARBON MONOXIDE & FORMIC ACID. |
| Heat of Combustion | -245.61 KJ/mol |
| Dissociation Constants | pKa 1: 1.46; pKa 2: 4.40 |
| Kovats Retention Index | 748 |
| Other Experimental Properties | MP: 101-102 °C GIVING OFF WATER OF CRYSTALLIZATION & STARTING TO SUBLIME; K1= 5.36X10-2; K2= 5.3X10-5; DENSITY: 1.653 @ 18.5 °C/4 °C; DENSITY OF 1% (WT/WT) AQ SOLN: 1.0035 @ 17.5 °C/4 °C; DENSITY OF 3% (WT/WT) AQ SOLN: 1.0105 @ 17.5 °C/4 °C; DENSITY OF 5% (WT/WT) AQ SOLN: 1.0175 @ 17.5 °C/4 °C; DENSITY OF 10% (WT/WT) AQ SOLN: 1.0350 @ 17.5 °C/4 °C; DENSITY OF 13% (WT/WT) AQ SOLN: 1.0455 @ 17.5 °C/4 °C /OXALIC ACID DIHYDRATE/ |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 90.03 g/mol |
|---|---|
| XLogP3 | -0.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 1 |
| Exact Mass | 89.99530854 g/mol |
| Monoisotopic Mass | 89.99530854 g/mol |
| Topological Polar Surface Area | 74.6 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 71.5 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
Description
Oxalic acid is a dicarboxylic acid with a chemical formula C2H2O4. It is also known as Ethanedioic acid or Oxidic acid. This organic compound is found in many vegetables and plants. It is the simplest dicarboxylic acid with condensed formula HOOC-COOH and has an acidic strength greater than acetic acid. It is hygroscopic and sensitive to heat. This compound may react violently with furfuryl alcohol, silver, sodium, perchlorate, sodium hypochlorite, strong oxidizers, sodium chlorite, acid chlorides, and alkali metals.
