CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Liquid |
|---|---|
| Density | 0.940 g/cu m @ 25 °C /Silicon fluid with 10 sq mm/s viscosity/ |
| Vapor Density | Pour point -73 °C; Electric Strength 1.4 KV/um; Electric Constant 2.60; Thermal conductivity at 65 °C = 1.3 W/(m.k). /Silicone fluid with 10 mm sq/5 viscosity/ |
| Vapor Pressure | 3.34 [mmHg] |
| Viscosity | 10 cu mm/S @ 25 °C /Silicone fluid with 10 mm sq/S viscosity/ |
| Surface Tension | 20.0 dyne/cm @ 25 °C /Silicone fluid with 10 mm sq/s viscosity/ |
| Refractive Index | Index of refraction 1.399 @ 20 °C/D /Silicone fluid with 10 mm sq/5 viscosity |
| Kovats Retention Index | 1031 1031 1034 1034 1038 1038 |
| Other Experimental Properties | Viscosity: 41 cSt at 37.8 °C /Silicone fluid with 10 mm sq/S viscosity/ |
| Chemical Classes | Metals -> Metalloid Compounds (Silicon) |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H226:Flammable liquids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P242:Use only non-sparking tools. P243:Take precautionary measures against static discharge. |
COMPUTED DESCRIPTORS
| Molecular Weight | 236.53 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 4 |
| Exact Mass | 236.10840961 g/mol |
| Monoisotopic Mass | 236.10840961 g/mol |
| Topological Polar Surface Area | 18.5 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 149 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
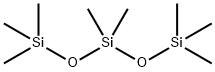
Octamethyltrisiloxane is an organosiloxane that is trisiloxane in which all the hydrogens have been replaced by methyl groups.