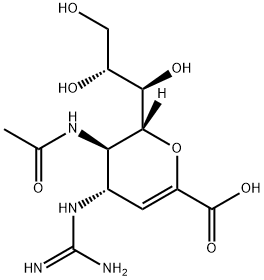
ZANAMIVIR HYDRATE
Synonym(s):4-Guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid;4-Guanidino-Neu5Ac2en;Zanamivir
- CAS NO.:139110-80-8
- Empirical Formula: C12H20N4O7
- Molecular Weight: 332.31
- MDL number: MFCD03791324
- EINECS: 691-117-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-06-30 15:45:59
What is ZANAMIVIR HYDRATE?
Absorption
Absolute bioavailability is very low following oral administration (2%). Following oral inhalation, bioavailability is 4% to 17%.
Description
Zanamivir was launched as Relenza in Australia for treatment of human influenza A and B virus infections. Zanamivir (4-guanidino-Neu5Ac2en) can be obtained by several similar ways, for instance in seven step synthesis starting from N-acetyI-D-neuraminic acid. Mechanistically, Zanamivir is a potent and specific inhibitor of neuraminidase (or sialidase), a key viral surface glycohydrolase essential for viral replication and disease progression by catalyzing the cleavage of terminal sialic acid residues from the glycoprotein. The in vitro activity of Zanamivir against a wide variety of influenza A and B strains was demonstrated in different model systems; its activity against clinically relevant isolates of influenza virus, with IC50 values ranging from 0.005 to 15 pM was superior to those of amantadine and rimantadine.
The Uses of ZANAMIVIR HYDRATE
ZANAMIVIR HYDRATE is a sialic acid analog that inhibits neuraminidase release of newly replicated influenza virus particles. It has been shown to selectively inhibit the growth of influenza A and B viruses in plaque reduction assays with IC50 values ranging from 5 to 14 nM and to directly inhibit influenza A and B virus neuraminidases with IC50 values ranging from 0.6 to 7.9 nM in vitro. The efficacy and tolerabilbity of zanamivir has also been established in clinical trial.[Cayman Chemical]
What are the applications of Application
Zanamivir sesquihydrate is a neuraminidase inhibitor
What are the applications of Application
Zanamivir is an antiviral agent and structural analog of sialic acid
Indications
For the prevention and treatment of influenza A and B.
Background
A guanido-neuraminic acid that is used to inhibit neuraminidase.
Pharmacokinetics
Zanamivir, an antiviral agent, is a neuraminidase inhibitor indicated for treatment of uncomplicated acute illness due to influenza A and B virus in adults and pediatric patients 7 years and older who have been symptomatic for no more than 2 days. Zanamivir has also been shown to significantly inhibit the human sialidases NEU3 and NEU2 in the micromolar range (Ki 3.7 +/-0.48 and 12.9+/-0.07 microM, respectively), which could account for some of the rare side effects of zanamivir.
Metabolism
Not metabolized
Properties of ZANAMIVIR HYDRATE
| Melting point: | 2560C (dec.) |
| Density | 1.75±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | H2O: soluble10mg/mL, clear |
| form | powder |
| color | white to beige |
Safety information for ZANAMIVIR HYDRATE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for ZANAMIVIR HYDRATE
| InChIKey | ARAIBEBZBOPLMB-UFGQHTETSA-N |
Abamectin manufacturer
New Products
3-N-BOC-(S)-AMINO BUTYRONITRILE 4-Piperidinopiperidine 2-Methyl-4-nitrobenzoic acid 2-(4-bromophenyl)-2-methylpropanoic acid 4-Acetyl-2-methylbenzoicacid Acetyl-meldrum's acid Ethyl-4-Pyrazole carboxylate 2,6 Di acetylpyridine 2,6-Pyridinedimethanol 5,7-Dichloro-3H-Imidazo[4,5-B]Pyridine 5-Bromo-2-Methoxy-4-Methyl-3-Nitropyridine 2-Fluoro-5-Iodopyridine 2-Fluoro-5-Methylpyridine 2-Chloro-3-Bromo-5-Amiopyridine METHYL-4-(BUTYRYLAMINO)3-METHYL-5-NITROBENZOATE TRANS-CYCLOBUTANE-1,2- DICARBOXYLIC ACID 5-Nitro indazole R-(-)-5-(2-AMINO-PROPYL)-2-METHOXY-BENZENESULFONAMIDE 1,3-cyclohexanedione 4-Aminophenaethylalchol (S)-(+)-4-BENZYL-2-OXAZOLIDINONE 3-NITRO-5-ACETYL IMINODIBENZYL 4-FLUORO PHENYL MAGNESIUM BROMIDE 1.0 M IN THF 1-HYDROXY-4-METHYL6-(2,4,4-TRI METHYL PHENYL)-2-PYRIDONE MONO ETHANOL AMINE(PIROCTONE OLAMINE)Related products of tetrahydrofuran
You may like
-
 Zanamivir 99%View Details
Zanamivir 99%View Details -
 139110-80-8 98%View Details
139110-80-8 98%View Details
139110-80-8 -
 2,3 Dichloro-4-Hydroxy Aniline 39183-17-0 99%View Details
2,3 Dichloro-4-Hydroxy Aniline 39183-17-0 99%View Details
39183-17-0 -
 17673-56-2 99%View Details
17673-56-2 99%View Details
17673-56-2 -
 13463-67-7 Titanium Dioxide 99%View Details
13463-67-7 Titanium Dioxide 99%View Details
13463-67-7 -
 143-07-7 99%View Details
143-07-7 99%View Details
143-07-7 -
 20776-67-4 99%View Details
20776-67-4 99%View Details
20776-67-4 -
 acid blue 113, acid navy blue , wool navy blue 0View Details
acid blue 113, acid navy blue , wool navy blue 0View Details
3351-05-1