Zalcitabine
Synonym(s):2′,3′-Dideoxycytidine;ddC
- CAS NO.:7481-89-2
- Empirical Formula: C9H13N3O3
- Molecular Weight: 211.22
- MDL number: MFCD00012188
- EINECS: 620-762-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-03-22 17:12:53
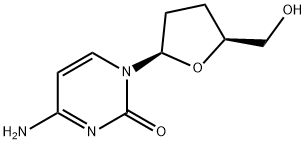
What is Zalcitabine?
Absorption
Bioavailability is over 80% following oral administration.
Toxicity
Acute overdose: Inadvertent pediatric overdoses have occurred with doses up to 1.5 mg/kg zalcitabine. Chronic overdose: in an initial dose-finding study in which zalcitabine was administered at doses 25 times (0.25 mg/kg every 8 hours) the currently recommended dose, one patient discontinued zalcitabine after 1? weeks of treatment subsequent to the development of a rash and fever.
Description
Zalcitabine is an orally active dideoxynucleoside andog for combination use with zidovudine in advanced HIV infection and also as monotherapy for AIDS patients who cannot tolerate or have not responded to zidovudine. It has a similar mechanism of action (inhibition of reverse transcriptase) to didanosine. Like didanosine, its side effect profile includes peripheral neuropathy. Unlike zidovudine, zalcitabine does not cause bone marrow suppression.
The Uses of Zalcitabine
A pyrimidine nucleoside analogue with antiviral activity
Background
A dideoxynucleoside compound in which the 3'-hydroxyl group on the sugar moiety has been replaced by a hydrogen. This modification prevents the formation of 5' to 3' phosphodiester linkages, which are needed for the elongation of DNA chains, thus resulting in the termination of viral DNA growth. The compound is a potent inhibitor of HIV replication at low concentrations, acting as a chain-terminator of viral DNA by binding to reverse transcriptase. Its principal toxic side effect is axonal degeneration resulting in peripheral neuropathy.
What are the applications of Application
2′,3′-Dideoxycytidine is an antiviral pyrimidine nucleoside analogue effective against HIV replication
Indications
For the treatment of Human immunovirus (HIV) infections in conjunction with other antivirals.
Pharmacokinetics
Zalcitabine is an analog of 2'-deoxycytidine that is pharmacologically related to but structurally different from other nucleotide reverse transcriptase inhibitors (NRTIs). Zalcitabine inhibits the activity of HIV-1 reverse transcriptase (RT) both by competing with the natural substrate dGTP and by its incorporation into viral DNA.
Metabolism
Zalcitabine is not reported to undergo significant hepatic metabolism; it is mainly phosphorylated intracellularly to zalcitabine triphosphate, the active substrate for HIV-reverse transcriptase. The concentration of zalcitabine triphosphate remains low for quantitative detection and measurement in the plasma following administration of therapeutic doses .
Properties of Zalcitabine
| Melting point: | 217-218 °C(lit.) |
| Boiling point: | 350.9°C (rough estimate) |
| Density | 1.2605 (rough estimate) |
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| solubility | DMSO (Slightly, Heated), Methanol (Slightly), Water (Slightly, Sonicated) |
| form | powder |
| color | colorless |
| Water Solubility | 5-10 g/100 mL at 19 ºC |
Safety information for Zalcitabine
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H351:Carcinogenicity |
| Precautionary Statement Codes |
P281:Use personal protective equipment as required. |
Computed Descriptors for Zalcitabine
| InChIKey | WREGKURFCTUGRC-POYBYMJQSA-N |
Abamectin manufacturer
ANAXLABORATORIES PRIVATE LIMITED
New Products
3-N-BOC-(S)-AMINO BUTYRONITRILE 4-Piperidinopiperidine 2-Methyl-4-nitrobenzoic acid 2-(4-bromophenyl)-2-methylpropanoic acid 4-Acetyl-2-methylbenzoicacid Acetyl-meldrum's acid Ethyl-4-Pyrazole carboxylate 2,6 Di acetylpyridine 2,6-Pyridinedimethanol 5,7-Dichloro-3H-Imidazo[4,5-B]Pyridine 5-Bromo-2-Methoxy-4-Methyl-3-Nitropyridine 2-Fluoro-5-Iodopyridine 2-Fluoro-5-Methylpyridine 2-Chloro-3-Bromo-5-Amiopyridine METHYL-4-(BUTYRYLAMINO)3-METHYL-5-NITROBENZOATE TRANS-CYCLOBUTANE-1,2- DICARBOXYLIC ACID 5-Nitro indazole R-(-)-5-(2-AMINO-PROPYL)-2-METHOXY-BENZENESULFONAMIDE 1,3-cyclohexanedione 4-Aminophenaethylalchol 3-NITRO-5-ACETYL IMINODIBENZYL (S)-(+)-4-BENZYL-2-OXAZOLIDINONE 4-FLUORO PHENYL MAGNESIUM BROMIDE 1.0 M IN THF 1-HYDROXY-4-METHYL6-(2,4,4-TRI METHYL PHENYL)-2-PYRIDONE MONO ETHANOL AMINE(PIROCTONE OLAMINE)Related products of tetrahydrofuran


You may like
-
 7481-89-2 Dideoxycytidine 98%View Details
7481-89-2 Dideoxycytidine 98%View Details
7481-89-2 -
 68915-31-1 99%View Details
68915-31-1 99%View Details
68915-31-1 -
 Azadirachtin 11141-17-6 99%View Details
Azadirachtin 11141-17-6 99%View Details
11141-17-6 -
 26062-79-3 99%View Details
26062-79-3 99%View Details
26062-79-3 -
 Geraniol 99%View Details
Geraniol 99%View Details
106-24-1 -
 BENZALKONIUM CHLORIDE BKC 99%View Details
BENZALKONIUM CHLORIDE BKC 99%View Details
8001-54-5 -
 Amrit Neem 11141-17-6 99%View Details
Amrit Neem 11141-17-6 99%View Details
11141-17-6 -
 Phosphonobutane Tri Carboxylic Acid 99%View Details
Phosphonobutane Tri Carboxylic Acid 99%View Details
37971-36-1