Titanium tetrachloride
Synonym(s):Titanium tetrachloride;Titanium(IV) chloride;TTC
- CAS NO.:7550-45-0
- Empirical Formula: Cl4Ti
- Molecular Weight: 189.68
- MDL number: MFCD00011267
- EINECS: 231-441-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-12-11 16:06:43
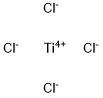
What is Titanium tetrachloride ?
Description
Titanium ore was first discovered in 1791 in Cornish beach
sands by an English clergyman, William Gregor. The actual
identification of the oxide was made a few years later by
a German chemist, M.H. Klaproth, who gave the metal
constituent of this oxide the name titanium, after the Titans of
Greek mythology. Pure metallic titanium was first produced in
the early 1900s in 1910 by M.A. Hunter at Rensselaer Polytechnic
Institute in cooperation with General Electric
Company.
Titanium tetrachloride is an inorganic compound that is an
important intermediate in the production of titanium metal
and the pigment titanium dioxide. On contact with humid air,
it forms opaque clouds of titanium dioxide (TiO2) and
hydrogen chloride (HCl). Early attempts to isolate titanium
metal from titanium tetrachloride were unsuccessful. The
process was improved and commercialized by William Kroll of
Luxembourg in the 1930s which involved the reduction of
titanium tetrachloride with magnesium in an inert gas atmosphere.
This process remains essentially unchanged today. The
primary use of titanium tetrachloride is for titanium dioxide
used in paints.
The production of titanium metal accounts for only 5% of
annual titanium mineral consumption, with the remainder
being used in the titanium pigment industry. Pigments are
produced using either a sulfate process or a more environmentally
acceptable carbochlorination process that converts
TiO2 into TiCl4. The latter process also supplies the TiCl4
necessary for the production of titanium metal.
The Uses of Titanium tetrachloride
Titanium tetrachloride is used as an intermediate in the
manufacture of titanium metal, titanium dioxide, titanous
chloride pigments, iridescent glass, and artificial pearls and as
a starting material for a variety of organic and inorganic titanium
compounds. It is also used as a dye, a polymerization
catalyst, and as a catalyst in many organic syntheses because of it acidity and oxophilicity in many applications in the chemical
industry. Titanium tetrachloride was formerly used as a smokeproducing
screen with ammonia for the military; however, due
to its extremely irritating and corrosive qualities in both liquid
and smoke formulation, military applications are rarely used.
The conversion of tetrachloride to titanium metal takes
place by the reduction of chloride with magnesium which
yields titanium metal and magnesium chloride and is referred
to as the Kroll process after its inventor:
2 Mg + TiCl4→2 MgCl2 + Ti
Properties of Titanium tetrachloride
| Melting point: | −25 °C(lit.) |
| Boiling point: | 135-136 °C(lit.) |
| Density | 1.73 g/mL at 20 °C(lit.) |
| Flash point: | 46 °F |
| storage temp. | Flammables area |
| solubility | H2O: soluble |
| form | Solution |
| appearance | Colorless liquid |
| color | Light yellow to dark brown |
| Water Solubility | reacts |
| Sensitive | Moisture Sensitive |
Safety information for Titanium tetrachloride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H330:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
Computed Descriptors for Titanium tetrachloride
Abamectin manufacturer
JSK Chemicals
Quantum Drugs Chemicals
Aquapharm Chemical Private Limited
New Products
3-N-BOC-(S)-AMINO BUTYRONITRILE 4-Piperidinopiperidine N-Benzyl-3-hydroxypiperidine 2-Methyl-4-nitrobenzoic acid 2-(4-bromophenyl)-2-methylpropanoic acid 4-Acetyl-2-methylbenzoicacid Acetyl-meldrum's acid Ethyl-4-Pyrazole carboxylate 2,6-Pyridinedimethanol 5,7-Dichloro-3H-Imidazo[4,5-B]Pyridine 5-Bromo-2-Methoxy-4-Methyl-3-Nitropyridine 2-Fluoro-5-Iodopyridine 2-Fluoro-5-Methylpyridine 2-Chloro-3-Bromo-5-Amiopyridine METHYL-4-(BUTYRYLAMINO)3-METHYL-5-NITROBENZOATE TRANS-CYCLOBUTANE-1,2- DICARBOXYLIC ACID 5-Nitro indazole R-(-)-5-(2-AMINO-PROPYL)-2-METHOXY-BENZENESULFONAMIDE 1,3-cyclohexanedione 4-Aminophenaethylalchol (S)-(+)-4-BENZYL-2-OXAZOLIDINONE 3-NITRO-5-ACETYL IMINODIBENZYL 4-FLUORO PHENYL MAGNESIUM BROMIDE 1.0 M IN THF 1-HYDROXY-4-METHYL6-(2,4,4-TRI METHYL PHENYL)-2-PYRIDONE MONO ETHANOL AMINE(PIROCTONE OLAMINE)Related products of tetrahydrofuran

You may like
-
 7550-45-0 Titanium(IV) chloride, 99% 99%View Details
7550-45-0 Titanium(IV) chloride, 99% 99%View Details
7550-45-0 -
 7550-45-0 98%View Details
7550-45-0 98%View Details
7550-45-0 -
 Titanium tetrachloride 99%View Details
Titanium tetrachloride 99%View Details
7550-45-0 -
 Titanium tetrachloride 7550-45-0 98%View Details
Titanium tetrachloride 7550-45-0 98%View Details
7550-45-0 -
 141-86-6 98%View Details
141-86-6 98%View Details
141-86-6 -
 4-Amino-2-Chloropyridine 14432-12-3 98%View Details
4-Amino-2-Chloropyridine 14432-12-3 98%View Details
14432-12-3 -
 13466-41-6 98%View Details
13466-41-6 98%View Details
13466-41-6 -
 Ammonium HexachloropalladateView Details
Ammonium HexachloropalladateView Details
19168-23-1