Tafluprost
- CAS NO.:209860-87-7
- Empirical Formula: C25H34F2O5
- Molecular Weight: 452.53
- MDL number: MFCD08062150
- EINECS: 234-199-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-05-14 17:56:40
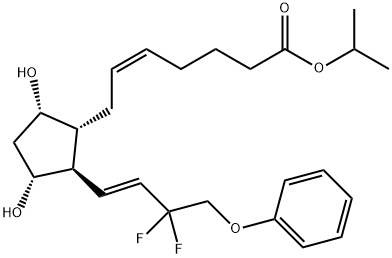
What is Tafluprost?
Absorption
Following instillation, tafluprost is absorbed through the cornea and is hydrolyzed to the biologically active acid metabolite, tafluprost acid. Tafluprost is an ester which makes the drug lipophillic enough to be quickly absorbed through. When administered to the eye, the peak plasma concentration (Cmax) and time to peak plasma concentration (Tmax) of tafluprost acid in healthy subjects was 26 pg/mL and 10 minutes respectively. a AUC, tafluprost acid = 394 pgmin/mL - 432 pgmin/mL.
Toxicity
Most common ocular adverse reaction is conjunctival hyperemia (range 4% – 20%).
Description
Glaucoma is second only to cataracts as a causative factor of blindness.
By 2010, it is estimated that approximately 60 million people worldwide
will be afflicted by glaucoma, so effective treatments should garner a large market.
PG analogs have been widely used for lowering IOP by increasing uveoscleral outflow through agonism of the prostanoid FP
receptor, and currently marketed versions include latanoprost, unoprostone isopropyl ester, bimatoprost, and travoprost.
Compared to the carboxylic acid of latanaprost
(Ki=4.7 nM), the carboxylic acid of tafluprost displayed a 10-fold greater
affinity for the prostanoid FP receptor (Ki=0.4 nM). The synthesis of
tafluprost begins with a Wittig condensation of the protected bicyclic
lactone carbaldehyde with a dimethyl phosphonate ketone derivative.
Compared to the carboxylic acid of latanaprost (Ki 4.7 nM), the carboxylic acid of tafluprost displayed a 10-fold greater affinity for the prostanoid FP receptor (Ki 0.4 nM). The synthesis of tafluprost begins with a Wittig condensation of the protected bicyclic lactone carbaldehyde with a dimethyl phosphonate ketone derivative. The bottom appendage is then completed by the fluorination of the ketone with morpholino-sulfur trifluoride. Hydrolysis of the benzoate ester protecting group liberates the hydroxy group, and reduction of the lactone is accomplished with aluminum hydride to generate the lactol. Condensation of this intermediate with the phosphonium salt of the acid side chain generates the free acid, or active ingredient, which is subsequently esterified with 2-iodopropane in the presence of DBU.
.
The Uses of Tafluprost
Tafluprost is a novel prostanoid used in the treatment of glaucoma and is the first prostanoid to be released in a preservative free-formula.
Background
A prostaglandin analogue ester prodrug used topically (as eye drops) to control the progression of glaucoma and in the management of ocular hypertension. Chemically, tafluprost is a fluorinated analog of prostaglandin F2-alpha. Tafluprost was approved for use in the U.S. on February 10, 2012.
Indications
Tafluprost is indicated for reducing elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension.
What are the applications of Application
Tafluprost is an FP receptor agonist
Pharmacokinetics
Tafluprost is a novel prostaglandin analog with a high affinity for the fluoroprostaglandin (FP) receptor PGF2α. Tafluprost has an affinity for the FP receptor that is approximately 12 times higher than that of the carboxylic acid of latanoprost, but with almost no potential to bind to other receptors.
Metabolism
Tafluprost is an ester prodrug which is rapidly hydrolyzed by corneal esterases to form its biologically active acid metabolite. Tafluprost acid is further metabolized via fatty acid β-oxidation and phase II conjugation into 1,2,3,4-tetranor acid.
Properties of Tafluprost
| Boiling point: | 552.9±50.0 °C(Predicted) |
| Density | 1.186 |
| storage temp. | 2-8°C |
Safety information for Tafluprost
Computed Descriptors for Tafluprost
Abamectin manufacturer
Honour Lab Limited
Aspen Biopharma Labs Pvt Ltd
New Products
3-N-BOC-(S)-AMINO BUTYRONITRILE 4-Piperidinopiperidine 2-Methyl-4-nitrobenzoic acid 2-(4-bromophenyl)-2-methylpropanoic acid 4-Acetyl-2-methylbenzoicacid Acetyl-meldrum's acid Ethyl-4-Pyrazole carboxylate 2,6 Di acetylpyridine 2,6-Pyridinedimethanol 5,7-Dichloro-3H-Imidazo[4,5-B]Pyridine 5-Bromo-2-Methoxy-4-Methyl-3-Nitropyridine 2-Fluoro-5-Iodopyridine 2-Fluoro-5-Methylpyridine 2-Chloro-3-Bromo-5-Amiopyridine METHYL-4-(BUTYRYLAMINO)3-METHYL-5-NITROBENZOATE TRANS-CYCLOBUTANE-1,2- DICARBOXYLIC ACID 5-Nitro indazole R-(-)-5-(2-AMINO-PROPYL)-2-METHOXY-BENZENESULFONAMIDE 1,3-cyclohexanedione 4-Aminophenaethylalchol (S)-(+)-4-BENZYL-2-OXAZOLIDINONE 3-NITRO-5-ACETYL IMINODIBENZYL 1-HYDROXY-4-METHYL6-(2,4,4-TRI METHYL PHENYL)-2-PYRIDONE MONO ETHANOL AMINE(PIROCTONE OLAMINE) 4-FLUORO PHENYL MAGNESIUM BROMIDE 1.0 M IN THFRelated products of tetrahydrofuran

You may like
-
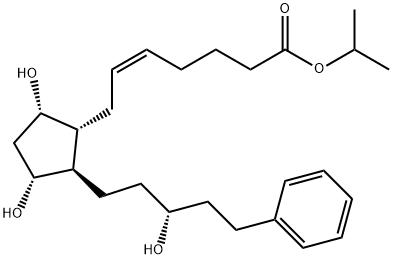
 209860-87-7 Tafluprost 98%View Details
209860-87-7 Tafluprost 98%View Details
209860-87-7 -
 209860-87-7 99%View Details
209860-87-7 99%View Details
209860-87-7 -
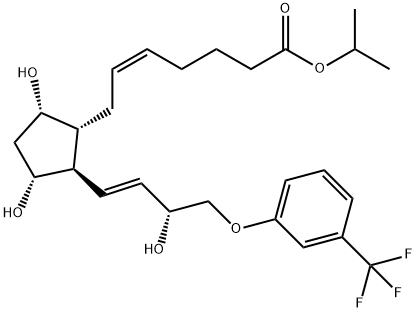
 209860-87-7 98%View Details
209860-87-7 98%View Details
209860-87-7 -
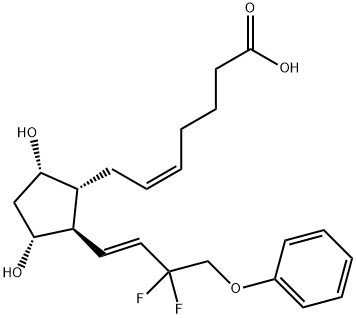
 209860-87-7 98%View Details
209860-87-7 98%View Details
209860-87-7 -
 17673-56-2 99%View Details
17673-56-2 99%View Details
17673-56-2 -
 13463-67-7 Titanium Dioxide 99%View Details
13463-67-7 Titanium Dioxide 99%View Details
13463-67-7 -
 143-07-7 99%View Details
143-07-7 99%View Details
143-07-7 -
 20776-67-4 99%View Details
20776-67-4 99%View Details
20776-67-4