Roflumilast
Synonym(s):3-(Cyclopropylmethoxy)-N-(3,5-dichloro-4-pyridinyl)-4-(difluoromethoxy)benzamide;3-Cyclopropylmethoxy-4-difluoromethoxy-N-[3,5-dichloropyrid-4-yl]-benzamide
- CAS NO.:162401-32-3
- Empirical Formula: C17H14Cl2F2N2O3
- Molecular Weight: 403.21
- MDL number: MFCD00938270
- EINECS: 685-382-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-09-07 14:58:50
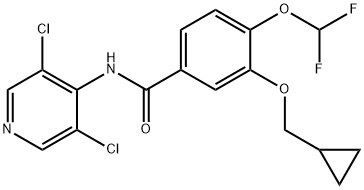
What is Roflumilast?
Absorption
After a 500mcg dose, the bioavailability of roflumilast is about 80%. In the fasted state, maximum plasma concentrations are reached in 0.5 to 2 hours, while in the fed state, Cmax is reduced by 40%, Tmax is increased by one hour, and total absorption is unchanged.
Applied topically, the mean systemic exposure for roflumilast and its N-oxide metabolite in adults was 72.7 ± 53.1 and 628 ± 648 h?ng/mL, respectively. The mean systemic exposure for roflumilast and its N-oxide metabolite in adolescents was 25.1 ± 24.0 and 140 ± 179 h?ng/mL, respectively.
Toxicity
There are no data regarding overdosage with orally administered roflumilast. Phase I studies in which roflumilast was administered at single doses up to 5000 mcg showed an increase in the incidence of headache, gastrointestinal disorders, dizziness, palpitations, lightheadedness, clamminess, and arterial hypotension. In the event of an overdose, administer support medical care as soon as possible. Hemodialysis is unlikely to be of benefit given the extensive protein binding of roflumilast.
Description
Roflumilast is a selective, orally active PDE4 inhibitor thatwas approved
in Germany in July 2010 as an add-on to bronchodilator treatment for
maintenance therapy of severe chronic obstructive pulmonary disorder
(COPD) associated with chronic bronchitis in adult patients with a history
of frequent exacerbations .
Roflumilast and its primary metabolite roflumilast N-oxide are potent and
competitive inhibitors of PDE4 and are equipotent against PDE4A, B, andD
but inactive against PDE4C and the other ten members of the PDE family
(PDEs 1–3, 5–11). Despite its inhibition of PDE4D (IC50=0.80 nM, N-oxide
IC50=2.0 nM), roflumilast shows the lowest incidence of nausea (3–5%)
among the PDE4 inhibitors investigated in clinical trials.Anti-inflammatory
effects of roflumilast have been demonstrated in preclinical cellular and
animal models. Roflumilast is synthesized in four steps from
3-(cyclopropylmethoxy)-4-hydroxybenzaldehyde. The difluoromethyl
ether is introduced by alkylation of the free phenolic group with
chlorodifluoromethane and base. The aldehyde moiety is oxidized to the
benzoic acid, which is then converted to an acid chloride and coupled with
3,5-dichloro-4-aminopyridine.
Roflumilast is rapidly absorbed and metabolized to its active metabolite,
roflumilast N-oxide. Metabolism is mediated by CYP3A4
and CYP1A2.
The Uses of Roflumilast
Roflumilast (Daxas) is a selective inhibitor of PDE4 with IC50 of 0.2-4.3 nM.
Indications
Oral roflumilast is indicated to reduce the risk of COPD exacerbations in patients with severe COPD associated with chronic bronchitis and a history of exacerbations.
Topical roflumilast is indicated to treat plaque psoriasis, including intertriginous areas, in patients 12 years of age and older.
Background
Roflumilast is a highly selective phosphodiesterase-4 (PDE4) inhibitor. PDE4 is a major cyclic-3',5′-adenosinemonophosphate (cyclic AMP, cAMP)-metabolizing enzyme expressed on nearly all immune and pro-inflammatory cells, in addition to structural cells like those of the smooth muscle or epithelium. The resultant increase in intracellular cAMP induced by roflumilast's inhibition of PDE4 is thought to mediate its disease-modifying effects, although its precise mechanism of action has yet to be elucidated.
The oral formulation of roflumilast is indicated to manage the chronic obstructive pulmonary disease. It was first approved by the EMA in July 2010, and by the FDA in January 2018. Roflumilast topical cream is indicated to treat plaque psoriasis. It was first approved by FDA in July 2022 and by Health Canada in April 2023.
What are the applications of Application
Roflumilast is a selective inhibitor of PDE4 (phosphodiesterase 4)
Pharmacokinetics
Roflumilast and its active metabolite, roflumilast N-oxide, increase cyclic adenosine-3′, 5′-monophosphate (cAMP) in affected cells by inhibiting PDE4. They are highly selective for PDE4 and are effectively inactive against PDEs 1, 2, 3, 5, and 7.
Metabolism
Roflumilast is metabolized to roflumilast N-oxide, the active metabolite of roflumilast in humans, by CYP3A4 and CYP1A2. The N-oxide metabolite is less potent than its parent drug in regards to PDE4 inhibition, but its plasma AUC is approximately 10-fold greater.
Properties of Roflumilast
| Melting point: | 158°C |
| Boiling point: | 430.6±45.0 °C(Predicted) |
| Density | 1.471±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMSO: soluble20mg/mL, clear |
| form | powder |
| color | white to beige |
Safety information for Roflumilast
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Roflumilast
Abamectin manufacturer
Micro Orgo Chem
Nivika Chemo Pharma Pvt Ltd
Bioaltus Laboratories Pvt Ltd
Navone Specialties (OPC) Pvt Ltd
New Products
3-N-BOC-(S)-AMINO BUTYRONITRILE 4-Piperidinopiperidine 2-Methyl-4-nitrobenzoic acid 2-(4-bromophenyl)-2-methylpropanoic acid 4-Acetyl-2-methylbenzoicacid Acetyl-meldrum's acid Ethyl-4-Pyrazole carboxylate 2,6 Di acetylpyridine 2,6-Pyridinedimethanol 5,7-Dichloro-3H-Imidazo[4,5-B]Pyridine 5-Bromo-2-Methoxy-4-Methyl-3-Nitropyridine 2-Fluoro-5-Iodopyridine 2-Fluoro-5-Methylpyridine 2-Chloro-3-Bromo-5-Amiopyridine METHYL-4-(BUTYRYLAMINO)3-METHYL-5-NITROBENZOATE TRANS-CYCLOBUTANE-1,2- DICARBOXYLIC ACID 5-Nitro indazole R-(-)-5-(2-AMINO-PROPYL)-2-METHOXY-BENZENESULFONAMIDE 1,3-cyclohexanedione 4-Aminophenaethylalchol (S)-(+)-4-BENZYL-2-OXAZOLIDINONE 3-NITRO-5-ACETYL IMINODIBENZYL 4-FLUORO PHENYL MAGNESIUM BROMIDE 1.0 M IN THF 1-HYDROXY-4-METHYL6-(2,4,4-TRI METHYL PHENYL)-2-PYRIDONE MONO ETHANOL AMINE(PIROCTONE OLAMINE)Related products of tetrahydrofuran
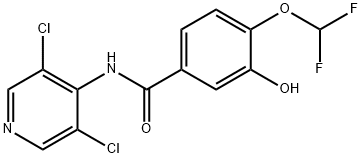
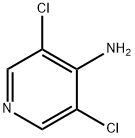
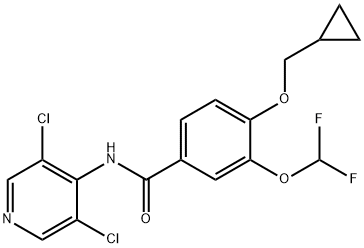
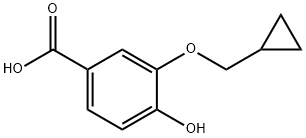
You may like
-
 162401-32-3 ROFLUMILAST 99%View Details
162401-32-3 ROFLUMILAST 99%View Details
162401-32-3 -
 162401-32-3 99%View Details
162401-32-3 99%View Details
162401-32-3 -
 Roflumilast 99%View Details
Roflumilast 99%View Details -
 Roflumilast 162401-32-3 98%View Details
Roflumilast 162401-32-3 98%View Details
162401-32-3 -
 162401-32-3 Roflumilast 98%View Details
162401-32-3 Roflumilast 98%View Details
162401-32-3 -
 162401-32-3 98%View Details
162401-32-3 98%View Details
162401-32-3 -
 Roflumilast 98%View Details
Roflumilast 98%View Details
162401-32-3 -
 Roflumilast Base 162401-32-3 99%View Details
Roflumilast Base 162401-32-3 99%View Details
162401-32-3