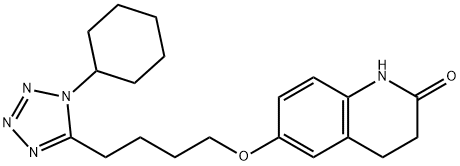
RDEA 594
- CAS NO.:878672-00-5
- Empirical Formula: C17H14BrN3O2S
- Molecular Weight: 404.28
- MDL number: MFCD22572730
- EINECS: 689-245-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-06-08 09:02:53
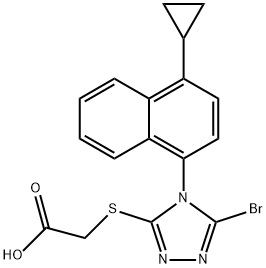
What is RDEA 594?
Absorption
Oral lesinurad is rapidly absorbed, reaching maximum plasma concentrations (Cmax) within 1–4 h following the administration a single 200 mg dose (in either the fed or fasted state).
Description
Approved by the FDA late in 2015, lesinurad is an urate anion exchange transporter 1 (URAT1) inhibitor for use in the treatment of gout. Ardea Biosciences, which is a subsidiary of AstraZeneca, developed lesinurad to be used in a combination therapy with xanthine oxidase inhibitors for the treatment of hyperuricaemia associated with gout. The approval process is ongoing in several other countries across the globe, with the EMA Committee for Medicinal Products for Human Use giving lesinurad a positive opinion for use as an adjunctive therapy in combination with xanthine oxidase inhibitors to treat hyperuricaemia.
The Uses of RDEA 594
Lesinurad is used to synthesize febuxostat which is a non-purine analog inhibitor of xanthine oxidase. Febuxostat is approved by the European Medicines Agency and the US Food and Drug Administration for treating gout.
Indications
For use, in combination with a xanthine oxidase inhibitor, for the treatment of hyperuricemia associated with gout in patients who have not achieved target serum uric acid levels with a xanthine oxidase inhibitor alone.
Background
Lesinurad is an oral uric acid transporter 1 (URAT1) inhibitor indicated for the treatment of hyperuricemia associated with gout. It reduces serum uric acid concentration through the inhibition of URAT1, an enzyme responsible for reuptake of uric acid from the renal tubule, and OAT4, another uric acid transporter associated with diuretic-induced hyperuricemia.
Marketed as the product Zurampic, it is indicated for use in combination with a xanthine oxidase inhibitor for the treatment of hyperuricemia associated with gout in
patients who have not achieved target serum uric acid levels with a xanthine oxidase inhibitor alone. In August 2017, a combination oral therapy consisting of lesinurad and Allopurinol was FDA-approved under the brand name Duzallo indicated for the treatment of gout-related hyperuricemia in patients with uncontrolled gout.
Pharmacokinetics
Dose-dependent reductions in serum uric acid levels and increases in urinary uric acid excretion have been observed following single and multiple oral doses of lesinurad.
Metabolism
Lesinurad undergoes oxidative metabolism mainly via the polymorphic cytochrome P450 CYP2C9 enzyme.
Properties of RDEA 594
| Melting point: | >171oC (dec.) |
| Boiling point: | 643.7±65.0 °C(Predicted) |
| Density | 1.72±0.1 g/cm3(Predicted) |
| storage temp. | -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly, Heated) |
| form | Solid |
| color | White to Off-White |
Safety information for RDEA 594
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for RDEA 594
Abamectin manufacturer
Honour Lab Limited
New Products
3-N-BOC-(S)-AMINO BUTYRONITRILE 4-Piperidinopiperidine 2-Methyl-4-nitrobenzoic acid 2-(4-bromophenyl)-2-methylpropanoic acid 4-Acetyl-2-methylbenzoicacid Acetyl-meldrum's acid Ethyl-4-Pyrazole carboxylate 2,6 Di acetylpyridine 2,6-Pyridinedimethanol 5,7-Dichloro-3H-Imidazo[4,5-B]Pyridine 5-Bromo-2-Methoxy-4-Methyl-3-Nitropyridine 2-Fluoro-5-Iodopyridine 2-Fluoro-5-Methylpyridine 2-Chloro-3-Bromo-5-Amiopyridine METHYL-4-(BUTYRYLAMINO)3-METHYL-5-NITROBENZOATE TRANS-CYCLOBUTANE-1,2- DICARBOXYLIC ACID 5-Nitro indazole R-(-)-5-(2-AMINO-PROPYL)-2-METHOXY-BENZENESULFONAMIDE 1,3-cyclohexanedione 4-Aminophenaethylalchol (S)-(+)-4-BENZYL-2-OXAZOLIDINONE 3-NITRO-5-ACETYL IMINODIBENZYL 4-FLUORO PHENYL MAGNESIUM BROMIDE 1.0 M IN THF 1-HYDROXY-4-METHYL6-(2,4,4-TRI METHYL PHENYL)-2-PYRIDONE MONO ETHANOL AMINE(PIROCTONE OLAMINE)Related products of tetrahydrofuran
You may like
-
 878672-00-5 Lesinurad 98%View Details
878672-00-5 Lesinurad 98%View Details
878672-00-5 -
 2,3 Dichloro-4-Hydroxy Aniline 39183-17-0 99%View Details
2,3 Dichloro-4-Hydroxy Aniline 39183-17-0 99%View Details
39183-17-0 -
 17673-56-2 99%View Details
17673-56-2 99%View Details
17673-56-2 -
 13463-67-7 Titanium Dioxide 99%View Details
13463-67-7 Titanium Dioxide 99%View Details
13463-67-7 -
 143-07-7 99%View Details
143-07-7 99%View Details
143-07-7 -
 20776-67-4 99%View Details
20776-67-4 99%View Details
20776-67-4 -
 acid blue 113, acid navy blue , wool navy blue 0View Details
acid blue 113, acid navy blue , wool navy blue 0View Details
3351-05-1 -
 Potassium OxonateView Details
Potassium OxonateView Details
2207-75-2