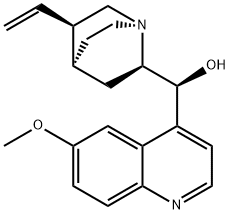
Quinine
Synonym(s):(-)-Quinine;6′-Methoxycinchonidine;6-Methoxy-α-(5-vinyl-2-quinuclidinyl)-4-quinolinemethanol;Quinine
- CAS NO.:130-95-0
- Empirical Formula: C20H24N2O2
- Molecular Weight: 324.42
- MDL number: MFCD00198096
- EINECS: 205-003-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-05-16 10:38:43
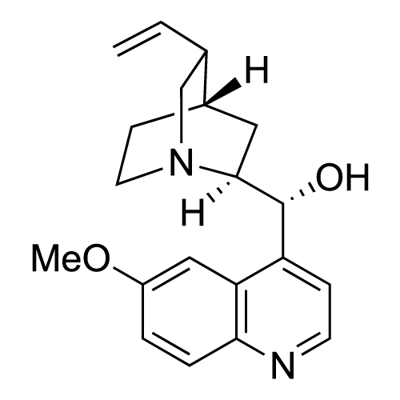
What is Quinine?
Absorption
76 - 88%
Toxicity
Quinine is a documented causative agent of drug induced thrombocytopenia (DIT). Thrombocytopenia is a low amount of platelets in the blood. Quinine induces production of antibodies against glycoprotein (GP) Ib-IX complex in the majority of cases of DIT, or more rarely, the platelet-glycoprotein complex GPIIb-IIIa. Increased antibodies against these complexes increases platelet clearance, leading to the observed thrombocytopenia.
Description
Quinine, was the first known antimalarial. It is a 4-quinolinemethanol derivative bearing a substituted quinuclidine ring. The use of quinine in Europe began in the seventeenth century, after the Incas of Peru informed the Spanish Jesuits about the antimalarial properties of the bark of an evergreen mountain tree they called quinquina (later called cinchona, after Dona Franciscoa Henriquez de Ribera [1576–1639], Countess of Chinchon and wife of the Peruvian Viceroy).
Description
Quinine, is an extract from the bark of the cinchona tree that has been used for more than three hundred years as an anti-malarial.?Woodward—Doering/Rabe—Kindler total synthesis?of quinine ushered in the modern era of synthetic organic chemistry. The first stereoselective synthesis was reported in 2001.
The Uses of Quinine
Because of its relatively constant and well-known fluorescence quantum yield, quinine is also used in photochemistry as a common fluorescence standard. It has been used for imaging of oxygen evolution and oxide formation. Chloride and bromide have been sh
Background
An alkaloid derived from the bark of the cinchona tree. It is used as an antimalarial drug, and is the active ingredient in extracts of the cinchona that have been used for that purpose since before 1633. Quinine is also a mild antipyretic and analgesic and has been used in common cold preparations for that purpose. It was used commonly and as a bitter and flavoring agent, and is still useful for the treatment of babesiosis. Quinine is also useful in some muscular disorders, especially nocturnal leg cramps and myotonia congenita, because of its direct effects on muscle membrane and sodium channels. The mechanisms of its antimalarial effects are not well understood.
Indications
For the treatment of malaria and leg cramps
What are the applications of Application
Quinine is an antimalarial
Pharmacokinetics
Quinine is used parenterally to treat life-threatening infections caused by chloroquine-resistant Plasmodium falciparum malaria. Quinine acts as a blood schizonticide although it also has gametocytocidal activity against P. vivax and P. malariae. Because it is a weak base, it is concentrated in the food vacuoles of P. falciparum. It is thought to act by inhibiting heme polymerase, thereby allowing accumulation of its cytotoxic substrate, heme. As a schizonticidal drug, it is less effective and more toxic than chloroquine. However, it has a special place in the management of severe falciparum malaria in areas with known resistance to chloroquine.
Metabolism
Hepatic, over 80% metabolized by the liver.
Properties of Quinine
| Melting point: | 173-175 °C(lit.) |
| Boiling point: | 462.75°C (rough estimate) |
| Density | 1.1294 (rough estimate) |
| Flash point: | >110°C (>230°F) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | H2O: soluble |
| form | Crystalline Powder |
| color | White |
| Water Solubility | slightly soluble |
| Sensitive | Light Sensitive |
Safety information for Quinine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P333+P313:IF SKIN irritation or rash occurs: Get medical advice/attention. |
Computed Descriptors for Quinine
Abamectin manufacturer
Medec Dragon Private Limited
New Products
3-N-BOC-(S)-AMINO BUTYRONITRILE 4-Piperidinopiperidine 2-Methyl-4-nitrobenzoic acid 2-(4-bromophenyl)-2-methylpropanoic acid 4-Acetyl-2-methylbenzoicacid Acetyl-meldrum's acid Ethyl-4-Pyrazole carboxylate 2,6 Di acetylpyridine 2,6-Pyridinedimethanol 5,7-Dichloro-3H-Imidazo[4,5-B]Pyridine 5-Bromo-2-Methoxy-4-Methyl-3-Nitropyridine 2-Fluoro-5-Iodopyridine 2-Fluoro-5-Methylpyridine 2-Chloro-3-Bromo-5-Amiopyridine METHYL-4-(BUTYRYLAMINO)3-METHYL-5-NITROBENZOATE TRANS-CYCLOBUTANE-1,2- DICARBOXYLIC ACID 5-Nitro indazole R-(-)-5-(2-AMINO-PROPYL)-2-METHOXY-BENZENESULFONAMIDE 1,3-cyclohexanedione 4-Aminophenaethylalchol 3-NITRO-5-ACETYL IMINODIBENZYL (S)-(+)-4-BENZYL-2-OXAZOLIDINONE 4-FLUORO PHENYL MAGNESIUM BROMIDE 1.0 M IN THF 1-HYDROXY-4-METHYL6-(2,4,4-TRI METHYL PHENYL)-2-PYRIDONE MONO ETHANOL AMINE(PIROCTONE OLAMINE)Related products of tetrahydrofuran


You may like
-
 130-95-0 Quinine 98%View Details
130-95-0 Quinine 98%View Details
130-95-0 -
 130-95-0 99%View Details
130-95-0 99%View Details
130-95-0 -
 Quinine, anhydrous 98%View Details
Quinine, anhydrous 98%View Details
130-95-0 -
 Quinine 130-95-0 98%View Details
Quinine 130-95-0 98%View Details
130-95-0 -
 130-95-0 98%View Details
130-95-0 98%View Details
130-95-0 -
 Quinine 130-95-0 99%View Details
Quinine 130-95-0 99%View Details
130-95-0 -
 130-95-0 Quinine 99%View Details
130-95-0 Quinine 99%View Details
130-95-0 -
 Quinine 130-95-0 99%View Details
Quinine 130-95-0 99%View Details
130-95-0